You’ll recall that my aim for 2024 is to rescue and revamp a good deal of old squamate-themed material from the Tet Zoo archives….
Caption: Smith’s African snake Grayia smythii (…. or should that be ‘smithii’?), photographed in the wild. When encountered by people, these animals will hiss and open the mouth in threatening fashion. The scales are smooth and the pupils are round. Image: Marius Burger, public domain (original here).
In previous articles – including those on garter snakes and house snakes and kin – we’ve looked at snakes conventionally lumped together within the old-fashioned, super-inclusive version of Colubridae. For the latter part of the 20th century at least, this group was interpreted as including basically all caenophidian snakes* that weren’t filesnakes, viperids or elapids. I like Gower et al.’s (2023) characterisation of this situation…
“Snake biologists understood that this was unsatisfactory, but they were somewhat overwhelmed by the morphological and ecological diversity of these many hundreds of species, and so put up with the situation while continuing their research” (p. 160).
* Caenophidia = so-called ‘higher snakes’, the vast assemblage that contains file snakes and Colubroides and excludes archaic Mesozoic snakes, the weird worm, thread and blind snakes, and the pythons and boas.
Caption: massively simplified phylogeny of crown snakes to show that Caenophidia is the giant clade that includes the youngest major snake groups. Elsewhere in the tree, studies continues to disagree on whether boas and kin form a clade with pythons and kin. The monophyly of Scolecophidia has also been challenged. Images: as ever, these are for the textbook. More on that here.
But as our understanding of caenophidian phylogeny, diversity and anatomy has improved (and as views on how taxonomy should reflect phylogeny have changed too), it’s become increasingly realized that ‘Colubridae’ of tradition should be split up. In part this is because it was polyphyletic, various of its constituent groups being closer to elapids (cobras, mambas and kin) than to the Eastern racer Coluber constrictor, the ‘core’ animal of Colubridae. And it’s also because the groups are sufficiently diverse – in all measures – to be considered ‘families’ all their own.
Introducing the grayiids. Here, we look at a poorly known group previously included within Colubridae as a ‘subfamily’. They’re often termed African water snakes (or watersnakes) and all species (five have been named to date) are included in the genus Grayia. Regarded as a subfamily within Colubridae, the group is thus Grayiinae. In taxonomic systems where Colubridae is restricted to the ‘Coluber group’ alone (and where what used to be Colubridae is Colubroidea), they’re Grayiidae, the grayiids.
Caption: big grayiids are said to be sluggish and even somewhat clumsy on land (the idea that they move “ponderously” was stated by Stephen Spawls and colleagues in 2002), whereas juveniles look to be agile, faster-moving animals. This juvenile G. tholloni was photographed in Togo; this species maintains banding into adulthood, even though the bands fade with age. Image: toganim, CC BY-NC (original here).
What’s with the origin of the scientific name? Grayia was first reported scientifically in 1818 when G. smythii was described from Democratic Republic of the Congo (DRC) as a species of Coluber – the concept of that genus was, at the time, substantially broader that it is today – by British zoologist William Elford Leach (1791-1836). That specimen had been collected in DRC on an expedition led by Captain J. K. Tuckey, and then studied by Leach back at the British Museum’s Natural History Department in London. Perhaps the most memorable detail of that expedition’s history, at least with respect to zoological specimens, is that three lion cubs were given to Tuckey and his men by local people. These cubs were “kept alive three days and fed on soaked bread, which doubtless caused their death” (Cranch in Tuckey & Christen 1818, p. 405). Anyway, you might know Leach best thanks to the giant New Caledonian gecko Rhacodactylus leachianus, named in his honour by Georges Cuvier in 1829, or by Leach’s storm petrel Hydrobates leucorhous.
Caption: taxa prominently associated with William Leach, rightly or not. At left, New Caledonian or Grande Terre giant gecko Rhacodactylus leachianus. At right, Leach’s storm petrel Hydrobates leucorhous. Images: Lennart Hudel, CC BY 4.0 (original here); Alexis Lours, CC BY 4.0 (original here).
In 1858, German-born British zoologist Albert Günther named a new snake that he regarded as worthy of its own genus: Grayia silurophaga, ‘Gray’s catfish eater’. This later proved synonymous with Coluber smythii… which thus became Grayia smythii. ‘Smythii’, incidentally, almost certainly honours Norwegian botanist Christen Smith (1785-1816) – he collected the type specimen on Tuckey’s expedition and somehow died on the same trip – and thus should really be ‘smithii’. Annoyingly, ‘smythii’ and ‘smithii’ are both used in the literature on this snake, as are their variants ‘smythi’ and ‘smithi’ (I’m sticking with smythii here as I still see it as the name that’s most prevalent in the literature). And, yes, it’s no secret that the reptiles (and other animals) of the global tropics sure are named after a whole lot of dead northern European white guys.
Caption: a hatchling G. smythii, photographed in the wild in eastern Congo. Juveniles of this species are conspicuously banded; adults are generally yellowish-brown, olive, or black with a mottled appearance where the cross-bars of juveniles have merged and ‘filled in’ the lighter areas. Image: (c) Kate Jackson.
So, what’s with the name ‘Grayia’? The British zoologist John Edward Gray (1800-1875) has been mentioned on numerous previous occasions here at Tet Zoo, often because he named the animals I write about (examples include arboreal alligator lizards Abronia and the whale Kogia). Well, Günther named Grayia specifically in his honour. Additional Grayia species were named during the 1860s and 90s: Caesar’s African water snake G. caesar by Günther in 1863*, the Ornate African water snake G. ornatus by José Vicente Barbosa du Bocage in 1866, and Tholloni’s African water snake G. tholloni by François Mocquard in 1897.
* Actually, he named it Xenurophis caesar. George Boulenger, in 1910, transferred it to Grayia.
Caption: John Edward Gray sure was a hard-working individual when it came to describing and naming animals that fell within his broad sphere of interest, which basically included all animals. He initially joined the British Museum’s Zoology Department to help catalogue reptiles, but ended up becoming Keeper of Zoology. At right are just two of the taxa he named: Kogia the whale and Abronia the lizard. Images (clockwise from left): public domain; Robert Pitman, in public domain (original here); Ethan Kocak, used with permission.
A phylogenetic analysis published this year (Chaney et al. 2024) found G. smythii and G. ornata to be sister taxa, and G. caesar and G. tholloni to also be sister taxa. They also found that these two clades diverged during the middle of the Oligocene (around 27 million years ago). That’s a deep divergence and this, combined with anatomical differences, led them to suggest resurrection of the name Xenurophis (originally applied to G. caesar), albeit as a subgenus within Grayia. Chaney et al. (2024) also found that Grayia included a hitherto overlooked distinct lineage that appeared to be cryptic species, and named G. obscura for a population from the Upper and Middle reaches of the Congo River and nearby. Spawls et al. (2018, p. 536) made reference to “one undescribed species” known from Cameroon, though I don’t know of its current status.
Incidentally, the common name ‘Caesar’s African water snake’ for G. caesar is fairly inappropriate since it seems that Günther named this snake in possible recognition of its magnificent, regal form (Beolens et al. 2011) (‘caesar’ being a name used for Imperial things thanks to its original association with the Roman emperor). A more accurate vernacular name would thus be ‘Magnificent African water snake’ or ‘Regal African water snake’ or such. I should also add that I find ‘African water snake’ clumsy and unlikeable – it’s just too generic – and would prefer it if we adopted an African name for the group. The local name dibomina is used for these snakes in many places, but one problem is that its plural is mabomina, which would be inconsistent with English conventions.
Caption: unfortunately, I don’t have any images of G. caesar that show how attractive and impressive it is in life, and this image of a dead one – presumably squished on a road – hardly does it justice. Image: (c) M. Cristina Carboni (original here).
Caption: Ornate African water snake G. ornata photographed in the wild. A prominently banded pattern is typical for this species and explains its vernacular English name. I find this snake to have a natricid-like demeanour and would suspect it to be a member of that group if I didn’t know better. Image: Marius Burger, CC0 (original here).
Some biology and natural history. Grayia snakes are robust and large, reaching 1.7 m and even 2.5 m in a few places in western Africa (Spawls et al. 2004). Relatively little is known about their natural history, they’re regarded as elusive, and they inhabit places – seasonal swamps and watercourses – where they can be hard to see and find. But as suggested by the common name, African water snakes are semi-aquatic and eat fish, frogs and tadpoles. A study of G. smythii in Nigeria demonstrated Tilapia and the catfish Clarias to be the main fish prey, with Xenopus tropicalis – tadpoles as well as metamorphs – being the frog the snakes ate the most (Godfrey & Luiselli 2001). This diet appears typical of G. smythii, at least, across its range.
Caption: everyone’s heard of Xenopus laevis, but less familiar (unless you’re a massive herp nerd) is the Western or Tropical clawed frog X. tropicalis. It’s generally smaller as an adult than X. laevis (with a snout to vent length of 3-5 cm) and often darker on its dorsal surface. Image: Václav Gvoždík, CC BY-SA 3.0 (original here).
Egg-laying happens during the winter dry season, and an unusual behavioural trait is that their eggs are deposited in several separate batches at two or three separate sites, an aspect of behaviour unknown for other snakes (Godfrey & Luiselli 2001). Nests are among leaf litter gathered between buttress roots and not located in an aquatic setting (I know that that would be highly unusual but it’s worth commenting on). Males and females are similar in size with males having proportionally longer tails, as is typical for snakes.
Until recently, I’d wrongly thought that these were animals of the Congo region alone. But they’re actually hugely widespread across continental Africa, with a range extending from Niger in the north to Angola in the south-west, and Kenya and Tanzania in the east. They occur, then, across a vast portion of one of the world’s greatest continents, albeit not in the far north or south, and not in the deserts.
Caption: a map of Africa, showing the countries from which four of the living grayiid species have been reported (so, it’s not really a range map, and it doesn’t include data from all recognized species). It’s messy given that some countries are inhabited by two or even three (like Cameroon, Angola and Democratic Republic of the Congo) grayiid species. With occurrences in nations like Chad, Ethiopia and Senegal, this group is not ‘Congolese’ only, even employing the most generous use of that term. Image: public domain.
Ethnozoological knowledge. A common complaint made about animals of all sorts is that it can be really hard, if not impossible, to find what local people – not explorers, naturalists or scientists from foreign, typically European, lands – know or think about them. The good news for Grayia is that a published study dedicated to local knowledge exists, albeit pertaining only to the Chaillu Massif of southern Gabon, and only to the Ornate African water snake G. ornata (Pauwels et al. 2002).
Known to Loumbou, Massango, Pounou and Nzebi people there as the dibomina (other local names exist too), the snake is regarded as non-venomous and as the “grandfather of all the other snakes” (Pauwels et al. 2002, p. 139). It’s widely eaten and regarded as a valued food item. Being aquatic, the snakes are most often captured in fishing nets but they’re also caught by hand in submerged burrows otherwise being investigated for catfish. In some places, G. ornata is also used to provide medical assistance to women in labour: the snake’s dried head is kept in a safe location, and water poured through it is drunk by the mother. Pauwels et al. (2002) explained that this connects the snake’s use of water as a refuge with the belief that the baby’s head will emerge faster than otherwise. Somewhat more magical beliefs in some places connect the application of fat from the snake with the attaining of improved swimming and fishing abilities.
Caption: it’s 2024, but we’re still at the point where you have to consult actual books made of paper to get good info on obscure animals like the snakes discussed here. Here are some (but not all) of the snake-themed books I checked while preparing this article. Image: Darren Naish.
On natural history, Grayia is known to be highly aquatic but also to be a good climber that drops into the water from overhanging branches when alarmed. It’s said to hunt underwater at night and a belief encountered in the Lunda area of Angola is that the snakes hunt in pairs (Pauwels et al. 2002). This is of special interest given suggestions that social hunting might be present in certain snake species. Otters are reported to eat Grayia trapped in nets, and the crocodiles Mecistops and Osteolaemus prey on them too (Pauwels et al. 2002). This is an impressive amount of information and an illustration of how much ethnozoological data can be collected if only researchers go to some trouble to collate and record it.
Caption: African crocodiles that can and do predate on grayiid snakes. At top, the slender snouted Mecistops (this individual, photographed in Tanzania, is supposedly M. leptorhynchus). Below, two different captive Osteolaemus, one formerly on show at Bristol Zoo and one formerly at Marwell Zoo. Osteolaemus is a species complex and working out which species captive specimens belong to is hard. Images: Leyo, CC BY-SA 3.0 (original here); Darren Naish.
Outside of ethnozoological data, Godfrey & Luiselli (2001) reported that herons and Nile monitors Varanus niloticus are also predators of these snakes and it’s been suggested that cobras – some of which forage aquatically – might also predate on them.
Where in phylogeny? Grayia has always been difficult to place phylogenetically. Though (as discussed above) conventionally lumped into the great, sprawling, traditional version of Colubridae and regarded as somewhat nondescript, it’s become more obvious as more evidence has come in that it’s unusual and distinctive. These are big and robust snakes relative to standard ‘colubrids’ and their semi-aquatic habits are consistent with views that aquatic habits might be archaic – more ‘ancestral’ – for colubroids. Hemipenis anatomy in snakes provides a great deal of phylogenetically important information and the hemipenis of Grayia differs importantly from that of most other colubroids: in many, the hemipenis is asymmetrical whereas it’s symmetrical and has a forked sulcus spermaticus in Grayia.
Add all of this together, and we have the idea of a distinct group that perhaps diverged early in evolutionary history from the lineage that includes most other colubroids. What has molecular data said on all this? The following area is a bit difficult to discuss given the competing taxonomic schemes used by different authors, so keep in mind that – from hereon – I’m using the system preferred by Zaher et al. (2009), where Colubridae is restricted to the Coluber clade, and several other supposed ‘colubrid subfamilies’ are elevated in rank.
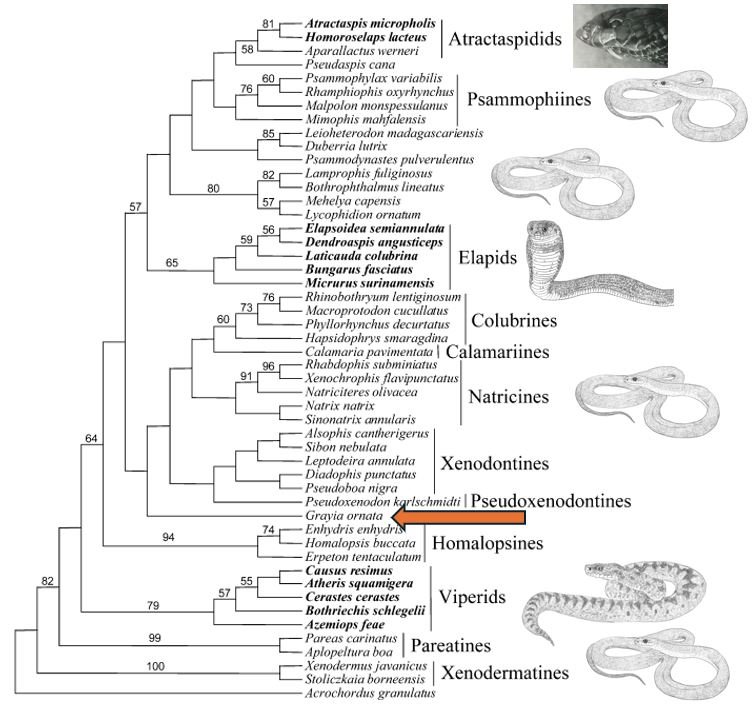
One of the first studies to analyse caenophidian snake phylogeny using genetics – they used one nuclear and three mitochondrial genes – was published by Vidal & Hedges (2002). And… newsflash: Grayia was the sister-taxon to virtually the whole of the rest of Colubroidea (the mudsnakes or Indo-Australian water snakes – the homalopsids – were shown diverging one node further down the tree), this implying that Grayia really should be imagined as the earliest-diverging, arguably ‘most archaic’, lineage within the whole group.
Caption: one of the preferred consensus phylogenetic trees from Vidal & Hedges (2002). Taxa ‘traditionally’ lumped into the highly inclusive late 20th century version of Colubridae are in disparate positions about the tree (they’re marked with the image of Coluber, the Eastern racer), and because viperids (marked with an image of an Adder Viperus berus), elapids (marked with a Naja cobra) and atractaspidids (marked with a photo of a burrowing asp/stiletto snake) are nested within this version of Colubridae, that version of Colubridae is paraphyletic, and here is part of the reason for its dissolution. Within the clade that includes colubrids in the most restrictive sense (shown here as colubrines), you can see that Grayia (red arrow) is sister to everything else. Image: Vidal & Hedges (2002); Darren Naish.
Kelly et al. (2003), just a little later, found Grayia to be an early-diverging lineage within a clade (let’s call it Clade X) that also included calamariids (reed snakes) and colubrids (Eastern racer and kin). This isn’t totally consistent with Grayia being an ‘early diverging colubroid’, however, since Clade X was sister to a Clade Y that included natricids (keelbacks, Natrix water snakes and kin) and xenodontids (reed snakes). These clades (X and Y) need names, by the way… hint hint. In the several molecular studies incorporating Grayia following that one, Grayia was specifically found to be part of the colubrid lineage (Pinou et al. 2004), the Asian vine snake* + colubrid clade (Lawson et al. 2005) or Natricidae (Kelly et al. 2009).
* This group has been called both Ahaetuliinae and Chrysopeleinae, with the latter having priority.
These diverse results are variable enough that, by around 2009-ish, there wasn’t a pinned down position on Grayia within Colubroidea, and it might have been best to regard it as incertae sedis. Zaher et al. (2009) thought that the balance of evidence made a position within Colubridae (remember: in the strict, narrowest sense) most likely. This emphatically refutes the idea that Grayia is ‘early diverging’ within Colubroidea as a whole. It’s deeply nested within the group, and in fact close to a clade generally regarded as one of the youngest within the whole assemblage.
A similar result was found by Pyron et al. (2011): a big Clade X that includes natricids and dipsadids is sister to Clade Y, and Grayia is again close to the base of Clade Y. But in a later and more comprehensive study, Pyron et al. (2013) found Grayia to be sister to the clade that includes Asian vine snakes and kin and Colubridae (again, meaning Eastern racer and kin). A similar position was also discovered by Figueroa et al. (2016) and Zaher et al. (2019).
Caption: a substantially simplified phylogenetic tree of colubrids and kin, based on the results of Figueroa et al. (2016), but using (where applicable) the taxonomy proposed by Zaher et al. (2009)… though this doesn’t quite work, since those two studies find very different positions for some of the relevant lineages (for Zaher et al. (2009), for example, hinge-toothed snakes or sibynophiines are within Colubrinae). The main point here is that grayiids are close to the restricted version of Colubridae. Images: Sibynophiinae: Thomas Brown, CC BY 2.0 (original here); Natricidae: Orchi, CC BY-SA 3.0 (original here); Pseudoxenodontidae: Umeshsrinivasan, CC BY-SA 3.0 (original here); Dipsadidae: Geoff Gallice, CC BY 2.0 (original here); Grayiidae: Kate Jackson, used with permission; Calamariidae: in public domain; Chrysopeleinae/Ahaetuliinae: Rushenb, CC BY-SA 4.0 (original here); Colubrinae: Dawson, CC BY-SA 2.5 (original here).
The conclusion has to be that, despite early indications (Vidal & Hedges 2002), Grayia isn’t especially ancient or ‘early diverging’ within Colubroidea after all. It’s instead part of a clade that includes the ‘core’ members of the whole lot, and any features that make it seem at all archaic are secondarily so and probably the result of specialisation for an unusual lifestyle.
And that about wraps everything up. A group of snakes that have long been obscure and enigmatic turn out to be reasonably well studied once you seek out the literature on them. And what were so often a sort of ‘footnote’ group that only get the briefest of mentions in discussions of colubroids can, in review texts of the future, now receive maybe a little more coverage.
For previous Tet Zoo posts on other squamates see…
In Quest of Anguids, May 2006
Pompey and Steepo, the World-Record-Holding Champion Slow-Worms, May 2007
Cambodia: now with dibamids!, May 2011
The Tet Zoo Guide to Mastigures, August 2018
The Remarkable Basilisks, May 2023
Do Lizards Really Have ‘Mite Pockets’?, March 2024
Meeting Lake Zacapu’s Garter Snake, March 2024
Ray Hoser, Number 1 Taxonomic Vandal, May 2024
The Mysterious Dibamids, May 2024
The Rehabilitation of Günther’s Black Cameroonian Snake, May 2024
Ikaheka and Other ‘Palatine Draggers’, Cryptozoic Elapid Snakes of Melanesia, June 2024
Arboreal Alligator Lizards of Mesoamerica... and Beyond!, July 2024
Refs - -
Beolens, B., Watkins, M. & Grayson, M. 2011. The Eponym Dictionary of Reptiles. Johns Hopkins University Press, Baltimore.
Chaney, T., Pauwels, O. S. G., Nagy, Z. T., Gvoždík, V., Kusamba, C., Badjedjea, G., Masudi, F. M., Akuboy, J. B., Ernst, R., Trape, J.-F., Chirio, L., Conradie, W., Keates, C., Wallach, V., Zassi-Boulou, A.-G., Vaughan, E. R. & Greenbaum, E. 2024. Phylogenetics and Integrative Taxonomy of African Water Snakes (Squamata: Colubridae: Grayia). Herpetological Monographs 38, 1-52.
Cranch, J. 1818. Appendix No. IV. A general notice of the animals taken by Mr John Cranch, during the expedition to explore the source of the River Zaire. In Tuckey, J. H. & Christen, S. Narrative of an Expedition to Explore the River Zaire, Usually Called the Congo, in South Africa, in 1816. J. Murray, London, pp. 407-419.
Godfrey, G. C. & Luiselli, L. 2001. Ecological studies on a population of the water snake Grayia smythii in a rainforest swamp of the Niger Delta, Nigeria. Contributions to Zoology 70, 139-146.
Gower, D., Garrett, K. & Maddock, S. 2023. Snakes: Their Diversity, Ecology and Behaviour. Natural History Museum, London.
Kelly, C. M. R., Barker, N, P. & Villet, M. H. 2003. Phylogenetics of advanced snakes (Caenophidia) based on four mitochondrial genes. Systematic Biology 52, 439-459.
Kelly, C. M. R., Barker, N. P., Villet, M. H. & Broadley, D. G. 2009. Phylogeny, biogeography and classification of the snake Superfamily Elapoidea: a rapid radiation in the late Eocene. Cladistics 25, 38-63.
Lawson, R., Slowinski, J. B., Crother, B. I. & Burbrink, F. T. 2005. Phylogeny of the Colubroidea (Serpentes): new evidence from mitochondrial and nuclear genes. Molecular Phylogenetics and Evolution 37, 581-601.
Pauwels O. S., Toham, A. K. & Mamonekene, V. 2002. Ethnozoology of the dibomina (Serpentes: Colubridae: Grayia ornata) in the Massif du Chaillu, Gabon. Hamadryad 27, 136-141.
Pinou, T., Vicario, S., Marschner, M. & Caccone, A. 2004. Relict snakes of North America and their relationships within Caenophidia, using likelihood-based Bayesian methods on mitochondrial sequences. Molecular Phylogenetics and Evolution 32, 563-574.
Pyron, R. A., Burbrink, F. T., Colli, G. R., Montes de Oca, A. N., Vitt, L. J., Kuczynski, C. A. & Wiens, J. J. 2011. The phylogeny of advanced snakes (Colubroidea), with discovery of a new subfamily and comparison of support methods for likelihood trees. Molecular Phylogenetics and Evolution 58, 329-342.
Spawls, S., Howell, K., Drewes, R. & Ashe, J. 2004. A Field Guide to the Reptiles of East Africa. A & C Black, London.
Vidal, N. & Hedges, S. B. 2002. Higher-level relationships of snakes inferred from four nuclear and mitochondrial genes. C. R. Biologies 325, 977-985.