An updated look at a very special group of remarkable burrowing snakes…
Caption: a fairly familiar portrait of a burrowing asp / stiletto snake that has appeared several times in the literature, and showing the right maxillary fang protruding while the mouth is closed. Image: I’m not sure of the origin of this image and will add credit info when I find it! I think it first featured in Underwood & Kovcha (1993).
Tet Zoo, the Squamate Years. In case it isn’t already obvious, one of my aims for 2024 is to release a lot of squamate-themed content here at Tet Zoo ver 4, and in part this involves rescuing and updating material previously published at ver 2 and ver 3. Today we’re going to look at a really fascinating group of caenophidian snakes called the burrowing asps or mole vipers.
The bulk of the text here was originally published at ver 2 (the ScienceBlogs years) back in May 2008, but – like all Sb material from that time – it’s since been removed or ruined. A wayback machine version does exist (here). Interesting discoveries have been made about burrowing asps since 2008, so I’ve made updates to the text where appropriate, and I’ve also updated it overall. Ok, let’s get to it.
Caption: if you’ve been following Tetrapod Zoology for a while, you might remember this very popular article from 2008. I know I go on about this a lot, but it’s a real source of frustration and disappointment to me that so much of my old Tet Zoo content is now unfindable except at wayback machine (which comparatively few people use or even know about). I see this as part of the larger, ‘dead internet’ problem that we now face as a society.
It goes without saying that most predatory animals need to open their mouths when they want to stab or bite potential prey items. But – get this – there’s a group of snakes that can erect their teeth and stab prey with a closed mouth. And that’s not all that’s interesting about these snakes. There are an awful lot of weird snakes, and one of my favourite groups is the atractaspidids (or atractaspids), and in particular the genus Atractaspis. These snakes are variously referred to as mole vipers, burrowing asps, burrowing adders, stiletto snakes or side-stabbing snakes. I’m going to (mostly) be referring to them as burrowing asps.
Burrowing asps are specialized for fossoriality (burrowing), with shiny-scaled, cylindrical bodies, small heads, a countersunk lower jaw, indistinct neck, short tail, and small eyes. Specialized fangs, a sophisticated venom apparatus and other characters show that they’re part of Colubriformes (sensu Zaher et al. 2009), the huge snake clade that includes viperids, elapids and that massive number of lineages traditionally grouped together as Colubridae.
Caption: good photos of burrowing asps in life aren’t all that common. Here’s a good one, showing a Southern stiletto snake A. bibronii, the type species for the genus. It occurs widely across the southern half of Africa. Note the protective or defensive pose, with the head held low and the tail tip raised high. Image: Ryan van Huyssteen, CC BY-SA 4.0 (original here).
Burrowing asps occur throughout much of Africa (with the exception of the north and south-west), and also the Sinai and Arabian peninsulas as far north as Israel. Around 22 species are currently recognized, but suspicion persists that cryptic species – those that look similar to others but are genetically very distinct from them – exist and await recognition. The type species for the genus is Bibron’s or the Southern stiletto snake A. bibronii, named by British zoologist Andrew Smith (who became Sir Andrew Smith in 1858) in 1849, the scientific name honouring his French colleague Gabriel Bibron (1806-1848). Species have been described at a fairly steady rate since then, the newest at the time of writing being Branch’s stiletto snake A. branchi of Liberia, named in 2019 (Rödel et al. 2019). These snakes are generally between 30 and 50 cm long. Oh, and they have a distinctive aromatic smell (Branch 1988). No-one knows why.
How to be a ‘fang stabber’. Burrowing asps have a formidable venom delivery apparatus. The maxillary fangs are massive, being about one-third as long as the whole skull, and the venom glands are enormous, extending into the neck and – in some species – continuing for approximating 20% of total body length (Underwood & Kochva 1993, Wollberg et al. 1998).
Caption: the burrowing asp skull – the lower of the two shown here – really is one of the most modified in all of Squamata. As is obvious if you compare it with the viperid skull shown at the top, the burrowing asp skull has a modified prefrontal (pf) and maxilla (mx), a startling lack of teeth, and a much pared down, reduced anatomy overall. Image: Deufel & Cundall (2006).
The rest of the dentition is highly reduced, there being just two short, gently curved dentary teeth and a couple of very small palatine teeth. The maxillary fangs (there are two in each maxilla, one of which is a replacement tooth kept in reserve) are huge compared to the short, block-like maxilla, and virtually its entire length is occupied by the transversely arranged fang sockets. The maxilla articulates with the relatively immobile prefrontal by way of a saddle-shaped joint, and this allows the maxilla to rotate. This is quite different from the condition present in viperids, where the articulatory surfaces between the maxilla and prefrontal are flat.
Caption: fang erection mechanism in Atractaspis, as illustrated by Deufel & Cundall (2003b). At left, it should be obvious that the articulation between the prefrontal (pf) and maxilla (mx), allow substantial rotation of the maxilla (and hence the giant fang(s) it houses). At right, the extreme shortness of the maxilla means that it can be rotated through a much larger angle than it can in more ‘normal’ snakes, like the Xenocalamus shown below. Figure from Deufel & Cundall (2003b).
Because both the maxillae and the fangs are directed posteriorly when at rest, and because the prefrontal doesn’t move much relative to the braincase, the maxilla-prefrontal unit can’t be thrown forwards to project the fangs anteroventrally (as happens in viperids and elapids).
The rotation of the maxilla is assisted by musculature attached to the slender, rod-like pterygoid-ectopterygoid unit. Usually in snakes, the pterygoid is attached to the palatine, but in burrowing asps the two are widely separated and only have a ligamentous connection (Underwood & Kochva 1993, Deufel & Cundall 2003a, b). This allows the pterygoid-ectopterygoid unit to swing antero-posteriorly without interference from the palatine. This has some implications, as we’ll see.
Caption: viperid, burrowing asp, and elapid palatal bones compared, from Deufel & Cundall (2006). The massive size of the burrowing asp fang (f) is obvious, as is the small size of the maxilla (mx). Note the slender, toothless pterygoid (pt) in the burrowing asp, and the lack of a bony connection (there’s a ligament there instead) between the pterygoid and palatine (pal). The reduced, bar-like form of the ectopterygoid (ec) in the burrowing asp is notable too. See Deufel & Cundall (2006) for a fuller explanation.
As the maxilla rotates ventrally, it opens up a slit along the mouth-line, providing enough space for the fang to protrude out of the mouth. The projecting tooth is then stabbed into prey with a swift posteroventral (down and backwards) jerk of the head. The snake might erect either the left-side fang or the right-side one: they don’t seem to deploy fangs from both sides at the same time (even though they probably could). When grasped behind the head in what would normally be regarded as a safe handling posture, a burrowing asp can – without opening its mouth – erect one of its super-long fangs and stab the hand of the person holding it. Kurnik et al. (1998) reported a case in which a herpetologist – specifically, one of the authors of the paper – was bitten on the finger by an Ein-Geddi burrowing asp A. engaddensis. “Local effects, oedema, erythema and numbness appeared within minutes, followed by systemic effects, including general weakness, sweating, pallor, fluctuations in the level of consciousness, vomiting and watery non-bloody diarrhoea. Gross oedema of the hand developed and extended up to the forearm” (Kurnik et al. 1998, p. 223). While the local effects healed within a few weeks, “some discoloration and tenderness remained even 10 months after”. Yikes. Don’t get bitten by a burrowing asp, that’s my advice.
And burrowing asp fangs aren’t just hollow cones, but (in all but two species) both canaliculate (that is, they house a tubular canal) and keeled along the posterior edges of their tips. This keel cuts into tissue when the snake stabs, presumably increasing the size of the wound and hence aiding the absorption of venom (Golani & Kochva 1988). It’s also been suggested that the keel helps the snake to yank its fangs out of its prey: while most long-fanged snakes strike at prey from a distance and only briefly engage with the prey, burrowing asps get right up close to prey before stabbing, sometimes stabbing several times. For this reason, Deufel & Cundall (2003b) recommended that a burrowing asp attack shouldn’t be referred to as a ‘strike’, but as a ‘fang stab’. When confronted with several prey items, burrowing asps have been reported to stab and envenomate several individuals before beginning to feed.
Specialized – but specialized for what? Given that burrowing asps do their fang stabbing in burrows and other confined spaces, you’d guess that all of these morphological and behavioural specializations have evolved to allow attacks where relatively little room for manoeuvring is possible. Who, or what, is getting stabbed? Atractaspis species prey on nestling mammals (mostly murids and shrews), and Deufel & Cundall (2003b) proposed that a reliance on such prey shaped their evolution, suggesting that “the success of [Atractaspis and relatives] is partly attributable to the use of the envenomation apparatus on mammals” (p. 58).
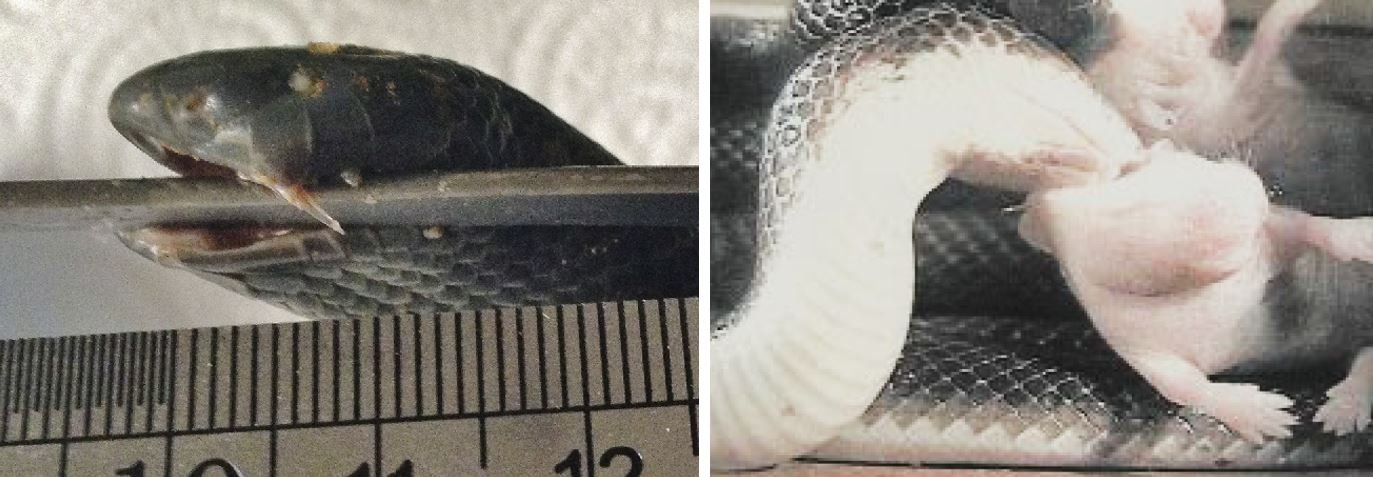
Caption: at left, another image showing how the maxillary fangs can be erected in a manner that’s very unusual relative to what’s more familiar. This is a preserved specimen of a Fat burrowing asp A. corpulenta. The snake is small, so that fang is only 4 mm long. At right, a photo from a sequence provided by Deufel & Cundall (2003b) where a Bibron’s burrowing asp A. bibronii is manipulating a rodent baby in preparation for swallowing. The snake has hooked a maxillary fang into the rodent and is using it as a gaff. Images: Deufel & Cundall (2003b); Tilbury & Verster (2016).
However, Shine et al. (2006) argued that mammals make up less than 25% of the diet of Atractaspis and drew attention to data showing that elongate fossorial squamates were the most important items in their diets. Attacking burrowing skinks and amphisbaenians within their burrows poses a problem, as the tails of these animals are similar in diameter to their bodies, making it difficult for an attacking snake to move past the tail and grab the body (you don’t want to grab the tail as both skinks and amphisbaenians are capable of autotomy).
Caption: amphisbaenians are one of my favourite groups of animals. We know for sure that snakes related to burrowing asps, like quill-snouted snakes (Xenocalamus, read on), are occasional predators of these dedicated burrowers. It’s thought that burrowing asps predate on them as well. The amphisbaenian portraits here show (at left) Amphisbaena of South America (an animal that’s therefore not relevant to burrowing asp predation) and Zarudny’s worm lizard Diplometopon zarudnyi of Iran, Iraq, the Arabian Peninsula and nearby. Images: Darren Naish.
Some burrowing asp specializations might, therefore, allow these snakes to push past the tail and envenomate or seize the prey’s body. Incidentally, Shine et al. (2006) noted that the ability to autotomise the tail among fossorial squamates might be an anti-atractaspidid adaptation, as a shed tail might both block the burrow and prevent venom injected into the tail from reaching the body. More study is needed to test this intriguing idea.
Being a good burrower makes a snake a poor swallower. The reduced palatal dentition and ligamentous linkage between the pterygoid and palatine raises an issue: how do these snakes transport prey within the mouth? Most snakes employ ‘pterygoid walking’, engaging the maxillary and pterygoid teeth on the left side with the prey and dragging it toward the throat, disengaging, and then doing the same with the maxillary and pterygoid teeth on the right, and so on. Burrowing asps can’t do that, since they sacrificed the ability to employ pterygoid walking when they de-coupled the palatine from the pterygoid and evolved a toothless pterygoid whose only function is to aid erection of the rotating maxilla and its fang. What, then, is a burrowing asp to do?
Caption: at left, Western Forest stiletto snake Atractaspis aterrima, quite possibly not in living state. At right, montage showing A. branchi, the most recently named member of the group (it was published in 2019). Images: Violette Dérozier, CC BY 4.0 (original here); Rödel et al. (2019), CC BY 4.0 (original here).
Deufel & Cundall (2003b) looked at this question. Firstly, burrowing asps sometimes used their super-long fangs as gaffs to manipulate prey into swallowing position. Movements of the maxilla and/or pterygoid are indeed not used in transporting prey, but by shifting the lower jaw posteroventrally, bending the anterior trunk region from side to side, and compressing and extending the neck, the snakes are able to move the mouth over the prey. They aren’t very good at it though, and take a long time to successful ingest prey (the account here is very much simplified: for the full story see Deufel & Cundall 2003b and Cundall & Deufel 2006).
The question of how burrowing asps transport prey within the mouth illustrates the point that these snakes have made a sort of trade-off in view of competing evolutionary pressures. To be a good burrower, and to function as a specialized fang stabber, Atractaspis has lost or modified some of the kinetic zones ordinarily present in the colubriform skull: the burrowing asp snout is relatively immobile relative to the braincase, for example, and the palatine doesn’t move with the pterygoid-ectopterygoid unit. But these modifications mean that burrowing asps have had to find other solutions to the problems posed by feeding, and in fact their solution is convergently similar to that evolved by some other fossorial snakes, like pipe snakes (Cylindrophis).
For more on this whole fascinating subject, be sure to check out the excellent papers published by Alexandra Deufel and David Cundall (Deufel & Cundall 2003a, b, 2006, Cundall & Deufel 2006). It should be obvious that I relied on them heavily in my discussion here.
Caption: the image of live burrowing asps we’ve seen so far make them look dark brown or nearly or entirely black. As this image of a Beaked burrowing asp A. duerdeni (of Namibia, Botswana and South Africa) shows, they’re sometimes marked with light, pinkish tones too. Image: Joubert Heymans, CC BY 4.0 (original here).
A controversial radiation. Finally, where do burrowing asps fit in phylogenetic terms? Their large venom glands, long, posteriorly inclined quadrates, vestigial or absent left lung, absent pelvis and many other details show that they’re part of the grand radiation formerly termed Colubroidea but better named Colubriformes (McDowell 1987, Lee & Scanlon 2002). I’m now following Zaher et al. (2009) in thinking that the term Colubroidea should be restricted to what was formerly ‘Colubridae’.
Burrowing asps were long regarded as viperids. However, Bourgeois (1961) proposed that they might be particularly closely related to another group of colubriforms, the aparallactines. Aparallactines are small-headed, fossorial snakes, often back-fanged. Some are blunt-snouted, others sharp-snouted. The ‘core’ member of this group is Aparallactus (the centipede-eaters), but it also includes the quill-snouted snakes (Xenocalamus), harlequin snakes (Homoroselaps) and purple-glossed snakes (Amblyodipsas). Molecular studies published within recent years have generally supported an atractaspidid – aparallactine alliance (Kraus & Brown 1998, Pyron et al. 2011, 2013, Figueroa et al. 2016, Zaher et al. 2019).
Caption: aparallactines are elusive, poorly known snakes, though there are certainly more images of them online today than there were when I first needed them back in 2008! Here’s a species that’s relatively well known within the group, the Slender quill-snouted snake Xenocalamus bicolor, one of five species within this endemic African genus. Image: Ryan van Huyssteen, CC BY-SA 4.0 (original here).
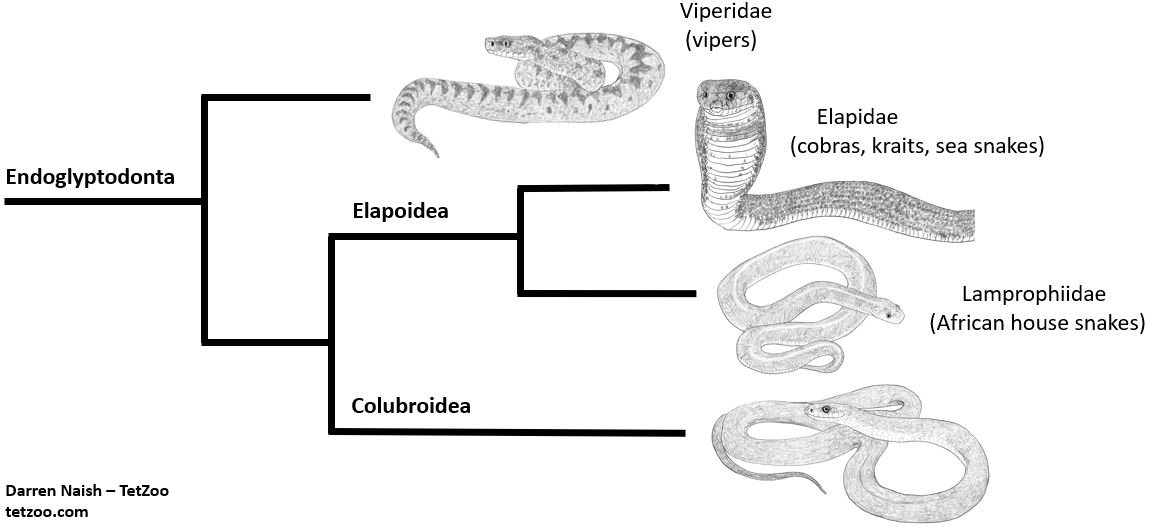
Molecular studies have also found this atractaspidid + aparallactine clade to be close to elapids (Kraus & Brown 1998, Pyron et al. 2011, 2013, Figueroa et al. 2016, Zaher et al. 2009, 2019). This atractaspidid, elapid and kin clade in turn belongs within Endoglyptodonta, and – within that – Elapoidea (Zaher et al. 2009). This accords with general thinking on where burrowing asps might belong based on anatomy. However, studies also find a number of other lineages to be even closer to burrowing asps than elapids are, among them the African house snakes and kin, or lamprophiines. Some studies have thus applied the name Lamprophiidae to the clade that includes lamprophiines, burrowing asps and various related group (e.g., Pyron et al. 2011, 2013, Figueroa et al. 2016). If this is followed, I shouldn’t be referring to ‘atractaspidids’… they are, instead, astractaspidine lamprophiids.
Other studies, however, do not find burrowing asps and kin to be so clearly nestled among lineages that might be united within that same version of Lamprophiidae, and instead have used a taxonomy where Atractaspididae is maintained as a distinct ‘family’-level group within Elapoidea (e.g., Zaher et al. 2009, 2019). This has implications for the taxonomy of various other, related snake groups too, but that’ll have to be a subject for another time. As ever, remember that the taxonomy we superimpose on top of the phylogeny is often up for debate, and still somehow subjective at some level.
Caption: a massively simplified depiction of phylogeny within the colubriform clade Endoglyptodonta. Burrowing asps are certainly elapoids, and within this clade are close to or part of Lamprophiidae. The topology shown here is consistent with several recent studies, but the taxonomy specifically follows that of Zaher et al. (2009). Image: this uses images created for the textbook I’m putting together. More on that on patreon.
That’s where we’ll end things for now. However… if you have any memory of the initial publication of this article at Tetrapod Zoology ver 2 back in 2008, you might recall that it resulted in a follow-up article discussing physical encounters – yes, I mean bites – with these snakes. It should republish that here as well, stay tuned…
For previous articles on squamates, see…
Cambodia: now with dibamids!, May 2011
The Tet Zoo Guide to Mastigures, August 2018
The Remarkable Basilisks, May 2023
Do Lizards Really Have ‘Mite Pockets’?, March 2024
Meeting Lake Zacapu’s Garter Snake, March 2024
Ray Hoser, Number 1 Taxonomic Vandal, May 2024
The Mysterious Dibamids, May 2024
Refs – -
Bourgeois, M. 1961. Atractaspis – a misfit among the Viperidae? News Bulletin of the Zoological Society of South Africa 3, 29.
Branch, B. 1988. Field Guide to the Snakes and Other Reptiles of Southern Africa. New Holland, London.
Cundall, D. & Deufel, A. 2006. Influence of the venom delivery system on intraoral prey transport in snakes Zoologischer Anzeiger – A Journal of Comparative Zoology 245, 193-210.
Deufel, A. & Cundall, D. 2003a. Prey transport in “palatine-erecting” elapid snakes. Journal of Morphology 258, 358-375.
Deufel, A. & Cundall, D. 2003b. Feeding in Atractaspis (Serpentes: Atractaspididae): a study in conflicting functional constraints. Zoology 106, 43-61.
Deufel, A. & Cundall D. 2006. Functional plasticity of the venom delivery system in snakes with a focus on the post-strike prey release behavior. Zoologischer Anzeiger 245, 249-267.
Golani, I. & Kochva, E. 1988. Striking and other offensive and defensive behavior patterns in Atractaspis engaddensis (Ophidia, Atractaspididae). Copeia 1988, 792-797.
Kraus, F. & Brown, W. M. 1998. Phylogenetic relationships of colubroid snakes based on mitochondrial DNA sequences. Zoological Journal of the Linnean Society 122, 455-487.
Kurnik, D., Haviv, Y. & Kochva, E. 1999. A snake bite by the burrowing asp, Atractaspis engaddensis. Toxicon 37, 223-227.
Lee, M. S. Y. & Scanlon, J. D. 2002. Snake phylogeny based on osteology, soft anatomy and ecology. Biological Reviews 77, 333-401.
McDowell, S. B. 1987. Systematics. In Seigel, R. A., Collins, J. T. & Novak, S. S. (eds) Snakes: Ecology & Evolutionary Biology. Macmillan (New York), pp. 3-49.
Pyron, R. A., Burbrink, F. T., Colli, G. R., Montes de Oca, A. N., Vitt, L. J., Kuczynski, C. A. & Wiens, J. J. 2011. The phylogeny of advanced snakes (Colubroidea), with discovery of a new subfamily and comparison of support methods for likelihood trees. Molecular Phylogenetics and Evolution 58, 329-342.
Shine, R., Branch, W. R., Harlow, P. S., Webb, J. K. & Shine, T. 2006. Biology of burrowing asps (Atractaspididae) from southern Africa. Copeia 2006, 103-115.
Tilbury, C. & Verster, J. 2016. A fatal bite from the burrowing asp Atractaspis corpulenta (Hallowell 1854). Toxicon 118, 21-26.
Underwood, G. & Kovcha, E. 1993. On the affinities of the burrowing asps Atractaspis (Serpentes: Atractaspididae). Zoological Journal of the Linnean Society 107, 3-64.
Wollberg, M., Kochva, E. & Underwood, G. 1998. On the rictal glands of some atractaspid snakes. Herpetological Journal 8, 137-143.
Zaher, H., Grazziotin, F. G., Cadle, J. E., Murphy, R. W., Cesar de Moura-Leite, J. & Bonatto, S. L. 2009. Molecular phylogeny of advanced snakes (Serpentes, Caenophidia) with an emphasis on South American xenodontines: a revised classification and descriptions of new taxa. Papéis Avulsos de Zoologia, Museu de Zoologia da Universidade de São Paulo 49, 115-153.