Today sees the publication of a major new technical paper by myself, Mark Witton and Liz Martin-Silverstone, titled ‘Powered flight in hatchling pterosaurs: evidence from wing form and bone strength’ and appearing in Scientific Reports (Naish et al. 2021). It’s OPEN ACCESS. Pass the champagne…
Caption: small juvenile and even hatchling pterosaurs - here, specimens of Pterodaustro guinazui from the Early Cretaceous of Argentina - were as flight capable as the much larger adults of their species. Pterodaustro is a mid-sized pterosaur with a wingspan of about 2.5 metres. Image: (c) Mark Witton.
TLDR: baby pterosaurs – even hatchlings – had wings fully specialised for active flight, were also excellent gliders, but almost certainly had very different lifestyles and occupied very different niches from their parents. That’s the basic summary of what we conclude, but here’s a slightly more detailed look…
For starters, it’s important to note that the idea that pterosaurs were able to fly very soon after (potentially immediately after) hatching is not at all new. This view has, in fact, been popular and well supported for a few decades now and has its strongest evidence in the fossils of very young juvenile pterosaurs, and even hatchlings still in their eggs. These have fully developed wings, adult-like proportions, and wing membranes similar in size and extent to those of their parents.
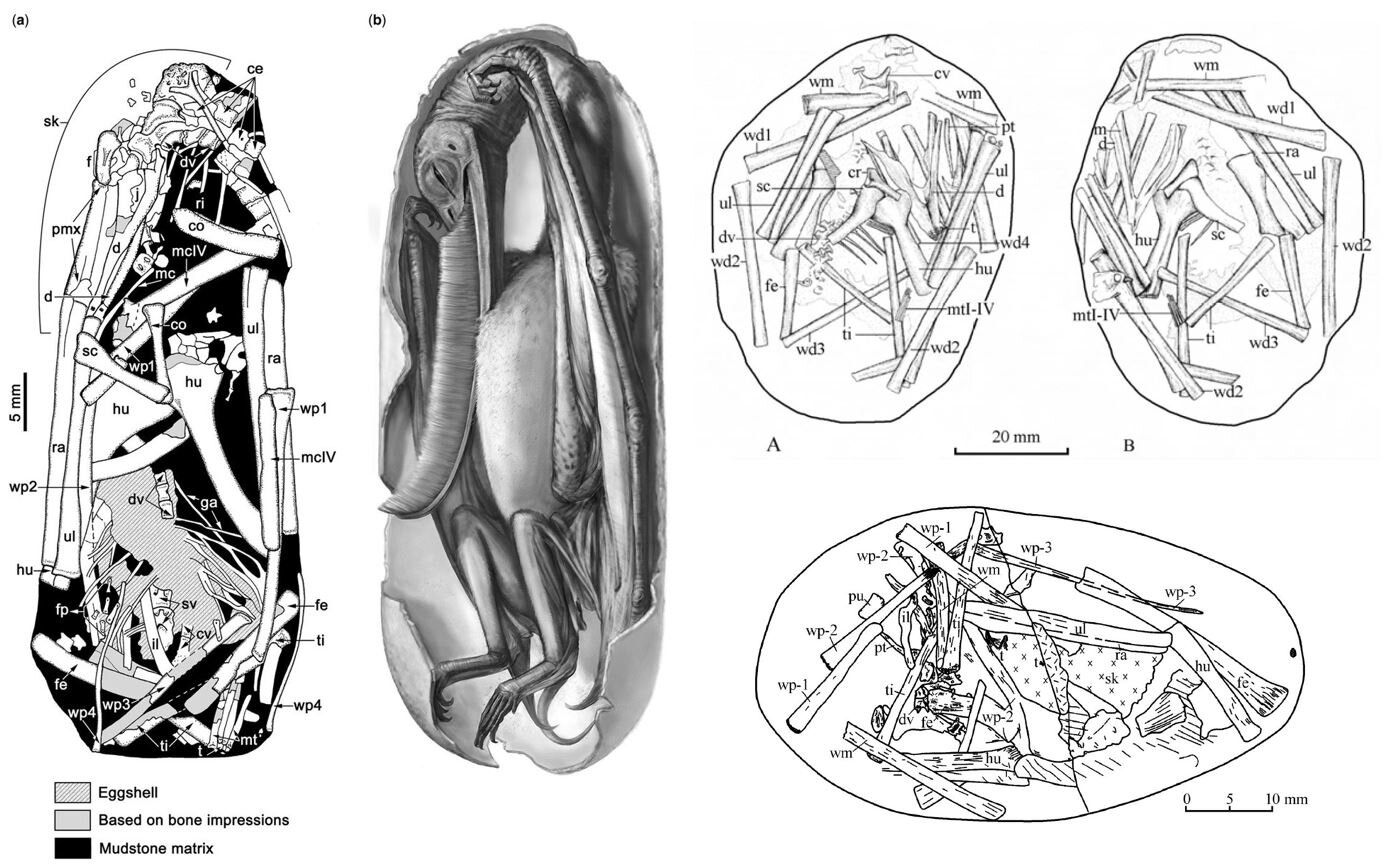
Caption: we don’t know a vast amount about pterosaur eggs and babies, but we at least know enough to see that embryos close to hatching had well-formed wing bones, the wings being of the size and shape consistent with proficient flight ability. At left, we see a diagram and life reconstruction showing an embryonic Pterodaustro., as illustrated by Codorniú et al. (2018). The two diagrams at upper right show the Yixian Formation embryo IVPP V-13758; and at lower right we see the embryo JZMO-03-03-2, also from the Yixian Formation. Images: Codorniú et al. (2018), Unwin et al. (2006).
The argument that pterosaurs were fully flight-capable at hatching has most prominently been advocated by David Unwin who (in his 2005 book The Pterosaurs from Deep Time) proposed the vernacular term flapling for flight-capable pterosaur babies (Unwin 2005). I and my colleagues have found this a useful term and have used it quite a lot. Birds that are capable locomotors within hours or minutes of hatching are termed precocial; those that are extreme in this regard (the mound-nesters or megapodes are exhibit A) are termed superprecocial, and Unwin’s model basically posits superprecociality in pterosaurs (Unwin 2005, Unwin et al. 2006, Unwin & Deeming 2008, 2019). Other pterosaur workers have presented similar views (e.g., Witton 2013, Bennett 2018, Hone et al. 2020).
This idea has, however, been challenged. In a 2012 study, Prondvai et al. argued that the babies of the Late Jurassic pterosaur Rhamphorhynchus were flightless at hatching, mostly climbed and did things on the ground as young juveniles, and only became flight-capable when at 50% or so of adult size (Prondvai et al. 2012). The 2017 announcement of eggs and embryos of the Cretaceous anhanguerian Hamipterus resulted in similar conclusions (Wang et al. 2017).
Caption: the discovery of numerous Hamipterus eggs (over 200) has led to popularisation of the idea that the babies of these pterosaurs were flightless and looked after by their parents. But read on… Image: Zhao Chuang.
What we have here are two competing models, one – the superprecocial one promoted by Unwin and others – saying that pterosaurs used what we might term a ‘flap-early’ strategy, and another – the one where hatchlings are flightless and flight abilities aren’t developed until later in life – that proposes a ‘fly-late’ strategy. A third possibility is implied in some writings in pterosaurs: that hatchlings were gliders but not active flapping fliers. We term this the ‘glide-early’ strategy.
Aware of these competing views and interested in evaluating them, myself, Mark and Liz set to work on a technical paper in which we pooled our thoughts on this topic, since we found that we’d all collected and analysed data relevant to the same issue.
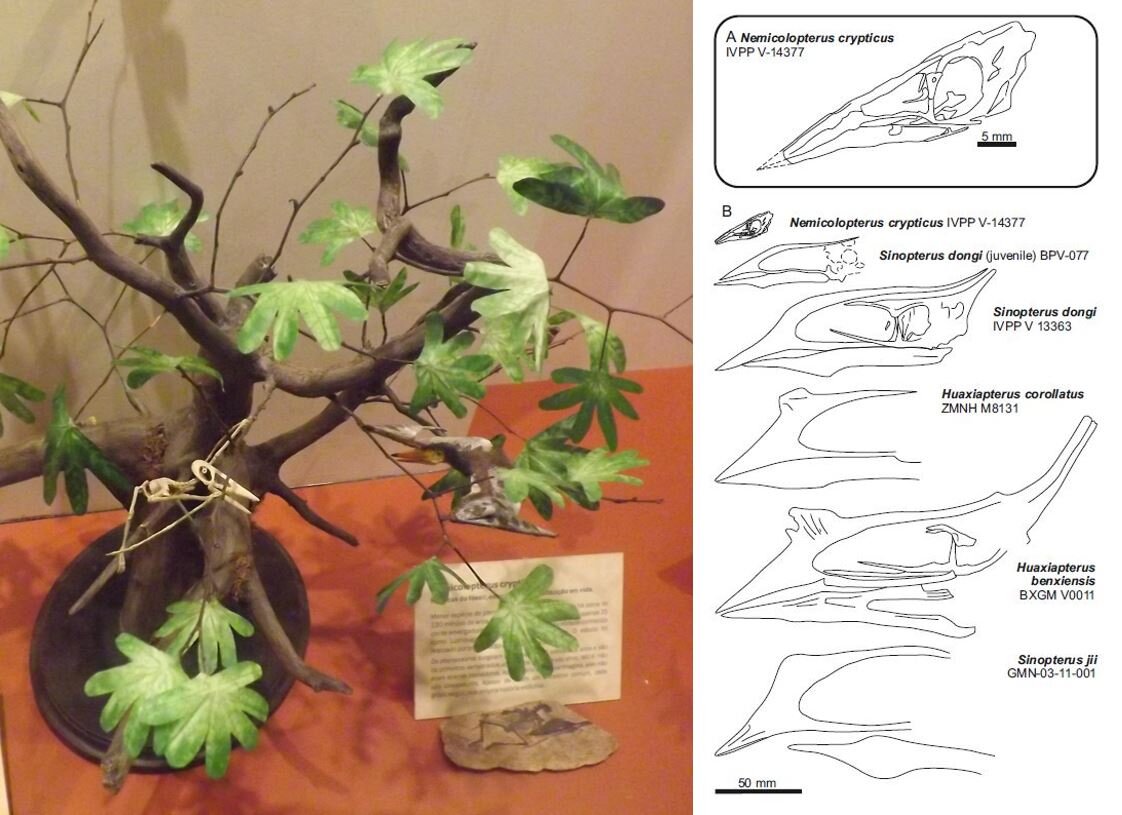
How many hatchling or very young juvenile pterosaurs are there for study? Well, not many. A substantial part of our paper concerns our justification for identifying Nemicolopterus crypticus from the Chinese Jiufotang Formation as a very young juvenile of the azhdarchoid Sinopterus, rather than as a distinct taxon that occupies a distinct position on the family tree. Readers with astronomical memories might remember me making this argument back in 2008 (here) when Nemicolopterus was first published. Mark followed up on this in his book (Witton 2013), but our new paper includes a more substantial analysis.
Caption: Brazil’s Museu Nacional in Rio de Janeiro (which I was lucky enough to visit in 2013) formerly included this model (at left), showing a reconstructed skeleton and life reconstruction of little Nemicolopterus. You can also see a replica of the actual specimen at lower right. At right, a diagram from Naish et al. (2021) in which Nemicolopterus is depicted as part of a possible growth sequence that includes specimens named Huaxiapterus and Sinopterus. Images: Darren Naish, Naish et al. (2021).
Evidence for strong flight abilities in baby pterosaurs. It should be obvious by now that several lines of evidence back up the view of flight-capable, superprecocial baby pterosaurs. But in order to actually test the three competing models of baby pterosaur behaviour, we used data from two specific areas: inferred gliding performance and bone strength. Our hypotheses were that gliding performance would demonstrate or refute aerial capability in even the youngest pterosaurs (if they were flightless as per the ‘fly-late’ model, their wings shouldn’t even permit gliding), and that bone strength would – again, in even the youngest pterosaurs – demonstrate or refute an ability to engage in true flapping flight.
Correlations between mass, wing area and glide performance – established across a list of modern animals but most usefully (for us) in the Draco lizards – mean that establishing the potential gliding performance of an animal isn’t difficult if you have the right data. If you know much about pterosaurs, you’ll know that some controversy exists over pterosaur mass and the relative size and extent of the wing membranes. I’m not going to discuss here why we went with the estimates we did, but we essentially used the work previously published by Witton (2008), Martin & Palmer (2014) and Elgin et al. (2011). The uncertainty that persists over wing membrane area means that we also used an estimate derived by making statistical generalisations about birds (Naish et al. 2021).
Caption: glide angles for assorted tetrapods, with hatchling pterosaurs at top. As should be obvious, hatchling pterosaurs out-perform living gliders. For full discussion see Naish et al. (2021).
The relationship between wing area and mass in hatchling and young juvenile pterosaurs means that they performed extremely well as gliders, out-gliding (in terms of distance travelled and how much height was lost across this distance) even the most adept of specialised living gliders. The wings of baby pterosaurs are more shaped for active sustained flight than specialised short-distance gliding (specialised short-distance, not-flapping gliders – Draco lizards, sugar gliders, flying squirrels and so on – have so-called low aspect wings, meaning that the wings are comparatively short and broad), so we’re not suggesting that babies relied on gliding more than on other aerial strategies (Naish et al. 2021). ‘Fly-late’ models which posit flightlessness in babies don’t, then, match what we know of juvenile pterosaur anatomy. And because the hatchlings and young juveniles are not built to be specialised gliders, we can also reject the ‘glide-early’ model (Naish et al. 2021). In short, our glide performance work provides strong support for the ‘flap-early’ model.
We also gleaned what data we could on the dimensions of juvenile pterosaur humeri, and then used these to calculate the relative failure force (or RFF) of these bones. We assumed that the bones of the juveniles had the same material properties as the bones of adults, something that looks reasonable but would benefit by confirmation in the future. What we found is that the humeri had extremely high RFFs, in cases being close to the highest RFFs of any pterosaur (Naish et al. 2021). Hold that thought.
Caption: the humeri (upper arm bones) of small and hatchling pterosaurs are thick and robust relative to their length, a fact consistent with a function in active flapping flight. For full discussion see Naish et al. (2021).
These strong humeri are very much indicative of a role in active flight. You might know that pterosaur humeral strength has also been linked to the role of this bone in forelimb-assisted launch (Habib 2008, 2013). It’s worth adding that the data we have from hatchlings shows that they too were capable of this strenuous activity. A final point worth noting is that the high strength of the hatchling humerus also makes it possible that these little animals were doing something especially strenuous or vigorous that we’ve so far not had reason to think about. We touch on this in the paper: possibilities include vertical springing (as in, a sort of leaping that doesn’t involve launching into flight) or digging (maybe in leaf litter rather than sediment).
Pterosaurs as niche fillers: the ecological picture. It’s not just that baby pterosaurs were good fliers, gliders and launchers right from hatching, they were also – very obviously – highly distinct from their parents in mass, wingspan and (purely by virtue of their size) their power to weight ratios, and hence aerodynamic abilities (Naish et al. 2021). What does this mean when it comes to pterosaur lifestyle and behaviour?
There are a number of possibilities here, and as usual we’re constrained by a lack of data. Could it be that baby pterosaurs lived with their parents in the same way that, say, baby gulls or shorebirds do? As we say in the paper (Naish et al. 2021), this is by no means impossible or implausible. When we consider the geological longevity and diversity of this group (pterosaurs were around for over 160 million years) and the diversity of behavioural strategies present in living animal groups, it might be that some pterosaur groups behaved this way while others did not.
Caption: in 2018, Chris Bennett described this partial juvenile Pteranodon specimen (FHSM 17956, representing the distal end of the radius and ulna [at far left), the wrist, and the proximal end of the wing finger). It would have had a wingpspan of about 1.8 metres, but was preserved in a marine environment also frequented by adults. The fact that Pteranodons this size and smaller are mostly absent from the relevant sediments indicates that juveniles inhabited different environments and occupied different niches. Image: Bennett (2018).
What appears more likely, however, is that parental care simply wasn’t needed and likely didn’t occur, the juveniles living an independent life in a totally different environment from that used by their parents.
Bennett proposed this for Pteranodon (Bennett 2018), arguing that juveniles inhabited inland environments, only later moving to oceanic habitats much later in life. We agree and go a bit further in arguing that this phenomenon – ontogenetic niche partitioning – was widespread and normal across pterosaurs. The fact that the wing proportions of juveniles allowed more dynamic flight and steeper climb abilities, coupled with their small overall size, could mean a reliance on more manoeuvrable prey and exploitation of cluttered, vegetated habitats unavailable to adults (Naish et al. 2021). In short, hatchlings and young juveniles likely occupied very different niches from older juveniles and especially from adults.
Caption: as pterosaurs moved through ontogeny, we surmise that they transitioned through several distinct niches. This idea is conveyed in this handy infographic produced by my coauthor Mark Witton: sorry it’s small and low-res here, see Naish et al. (2021) for better quality.
The picture that emerges here is one similar to that established for some dinosaurs. If the juveniles of big-bodied species were occupying distinct ecological niches from their parents, they were essentially functioning as ‘different species’. The result: one species functions as ‘several’ across its lifespan, is potentially better at exploiting niches and resources across its environment than would a species with a more conserved niche, and a low number of species (potentially even one) can occupy the niches used by several or many species in another animal group. You may already have heard of dinosaur-based studies that posit big theropods (specifically tyrannosaurids) as similar ‘niche fillers’, their juveniles apparently able to occupy ecological space ‘ordinarily’ used by species with smaller adult body sizes. Well, it might be that the same was true for pterosaurs too.
Caption: live with your baby, look after your baby, feed on the same stuff as your baby? That’s not niche partitioning. So… the sort of things that mammals (like these wildebeest) do is pretty much the opposite of what pterosaurs did. You should imagine pterosaurs as being more like fish or amphibians than mammals, at least when it comes to niche occupation across ontogeny. Image: CC BY-SA 2.0, Joachim Huber (original here).
The slow crawl and mishaps of completing a manuscript. There’s one final thing I want to say about this paper, and it’s more of a personal thing than a scientific one. It’s boring so you might want to skip it. This being that it took literally years to put this paper together. It actually has a ridiculous backstory that goes all the way back to 2008. We finally submitted the manuscript in early 2019, and review had been completed within several months. The problem with doing science in your ‘spare time’ (neither myself, Mark or Liz are salaried research scientists: any scientific writing or research we do happens in ‘spare time’), is that it’s very hard to find time to do things like deal with the rewrites and new tests demanded by peer review. Having said that, we did jump through the required hoops eventually but weren’t able to produce a revised version until March 2021. So – wow, talk about slow work.
Some of the hiatuses in our work resulted in us missing pieces of literature that were published in the interim, in particular Unwin & Deeming’s 2019 ‘Prenatal development in pterosaurs and its implications for their postnatal locomotory ability’. This is frustrating as that paper arrives at conclusions highly similar to our own (Unwin & Deeming 2019, Naish et al. 2021); furthermore, our missing of it makes it looks as if we deliberately snubbed or ignored it. We didn’t: we simply missed it.
Caption: the ‘flapling’ hypothesis mostly (…. mostly) has its genesis in Unwin’s 2005 The Pterosaurs from Deep Time. The work on pterosaur precociality and niche partitioning that you’ve been reading about here means that scenes like that depicted by John Sibbick on the cover of Peter Wellnhofer’s The Illustrated Encyclopedia of Pterosaurs (first published in 1991) are best regarded as technically wrong.
I’ve been alerted to some other oversights. For one thing, I (and I say ‘I’ because I’m solely responsible for this specific detail) screwed up on the referencing, the result being that there are a few places in the discussion section where I wrongly attribute specific statements and views to the wrong author. We’ve contacted the journal about this (thanks to David Unwin for bringing it to our attention) and, while we discovered it too late in order to get it changed in the published version of the paper, we’re having a correction published in the near future.
Anyway, it’s good to see this work finally in print. As I’ve hopefully emphasised sufficiently well in this article, we are most certainly not the first to talk about pterosaur superprecociality or ontogenetic niche partitioning and indeed we’re agreeing with the arguments made previously by other authors (Unwin 2005, Bennett 2018, Unwin & Deeming 2019, Hone et al. 2020). The novelty of our study, instead, is that we use specific tests to better establish these models over others that have been presented, and that we propose more finely honed ideas on the ways in which hatchlings and young juveniles might have differed from older juveniles and adults (Naish et al. 2021). I think it’s an exciting view of pterosaur biology, and future finds and additional work will reveal whether it can be better supported.
For previous TetZoo articles on pterosaurs and their biology and diversity, see…
Tiny pterosaurs and pac-man frogs from hell, March 2008
Daisy’s Isle of Wight Dragon and why China has what Europe does not, March 2013
In Rio for the 2013 International Symposium on Pterosaurs, May 2013
Quetzalcoatlus: the evil, pin-headed, toothy nightmare monster that wants to eat your soul, August 2013
Were azhdarchid pterosaurs really terrestrial stalkers? The evidence says yes, yes they (probably) were, December 2013
Some Azhdarchid Pterosaurs Were Robust-Necked Top-Tier Predators, February 2017
Postcranial Palaeoneurology and the Lifestyles of Pterosaurs, August 2018
New Perspectives on Pterosaur Palaeobiology, the TetZoo Review, September 2020
Refs - -
Bennett, S. C. 2018. New smallest specimen of the pterosaur Pteranodon and ontogenetic niches in pterosaurs. Journal of Paleontology 92, 254-271.
Codorniú, L., Chiappe, L. & Rivarola, D. 2018. Neonate morphology and development in pterosaurs: evidence from a ctenochasmatid embryo from the Early Cretaceous of Argentina. In Hone, D. W. E., Witton, M. P. & Martill, D. M. (eds) New Perspectives on Pterosaur Palaeobiology. Geological Society, London, Special Publcations, pp. 83-94.
Habib, M. B. 2008. Comparative evidence for quadrupedal launch in pterosaurs. Zitteliana B28, 159-166.
Habib, M. 2013. Constraining the air giants: limits on size in flying animals as an example of constraint-based biomechanical theories of form. Biological Theory 8, 245-252.
Hone, D. W. E., Ratcliffe, J. M., Riskin, D. K., Hermanson, J. W., & Reisz, R. R. 2020. Unique near isometric ontogeny in the pterosaur Rhamphorhynchus suggests hatchlings could fly. Lethaia 54, 106-112.
Martin, E. G., & Palmer, C. A. 2014. novel method of estimating pterosaur skeletal mass using computed tomography scans. Journal of Vertebrate Paleontology 34, 1466-1469.
Unwin, D. 2005. The Pterosaurs from Deep Time. Pi Press, New York.
Unwin, D. M. & Deeming, D. M. 2008. Pterosaur eggshell structure and its implications for pterosaur reproductive biology. Zitteliana B28, 199-207.
Unwin, D. M. & Deeming, D. M. 2019. Prenatal development in pterosaurs and its implications for their postnatal locomotory ability. Proceedings of the Royal Society B 286, 20190409.
Unwin, D. M., Lü, J. & Deeming, D. C. 2006. Were all pterosaurs oviparous? In Lü, J. C., Kobayashi, Y., Huang, D. & Lee, Y.-N. (eds) Papers from the 2005 Heyuan International Dinosaur Symposium. Geological Publishing House (Beijing), pp. 141-167.
Wang, X., Kellner, A. W. A., Jiang, S., Cheng, X., Wang, Q., Ma, Y., Paidoula, Y., Rodrigues, T., Chen, H., Sayão, J. M., Li, N., Zhang, J., Bantim, R. A. M., Zhang, X., Qiu, R. & Zhou, Z. 2017. Egg accumulation with 3D embryos provides insight into the life history of a pterosaur. Science 358, 1197-1201.
Witton, M. P. 2008. A new approach to determining pterosaur body mass and its implications for pterosaur flight. Zitteliana B28, 143-158.