It’s time once more to visit the amazing world of squamates, and again we’re looking at snakes. Today: the extremely obscure Small-eyed or Ikaheka snake of New Guinea and some of the surrounding islands. What’s the deal with this unusual animal?
Caption: a very glossy, clean Small-eyed snake photographed in Karkar Island, New Guinea in 2010. There’s an almost iridescent sheen to some of the scales. Image: Wolfgang Wüster, used with permission.
Known technically as Micropechis ikaheka, the Small-eyed snake is stocky and medium-sized, reaching 2 m in total. Its unusually small eyes are among its most distinctive features, and indeed it’s sufficiently unusual in anatomy that it’s the only (currently) recognized member of its genus. Incidentally, the name ‘Small-eyed snake’ is also sometimes used for Cryptophis nigrescens, an east Australian species more commonly called the Eastern small-eyed snake to remove confusion.
Little has been published on Micropechis in total but – unlike many of the snakes covered at Tetrapod Zoology in the past – comparatively little has been published on it since I first wrote about, that being back in 2010 when it was covered at ver 2 (the wayback version of that article is here).
Caption: yes, this is another ‘old’ Tet Zoo article – again from ver 2, the ScienceBlogs years – that I’ve now rescued and resuscitated. At right: I’m a big fan of the work and writings of Dr Mark O’Shea; all of his books are excellent. This is the cover of the second, 2011 edition of his Venomous Snakes of the World, and… yes it does include a section on Micropechis. As ever, good images of the cover online are hard to find, so this is a photo of my own (signed) copy.
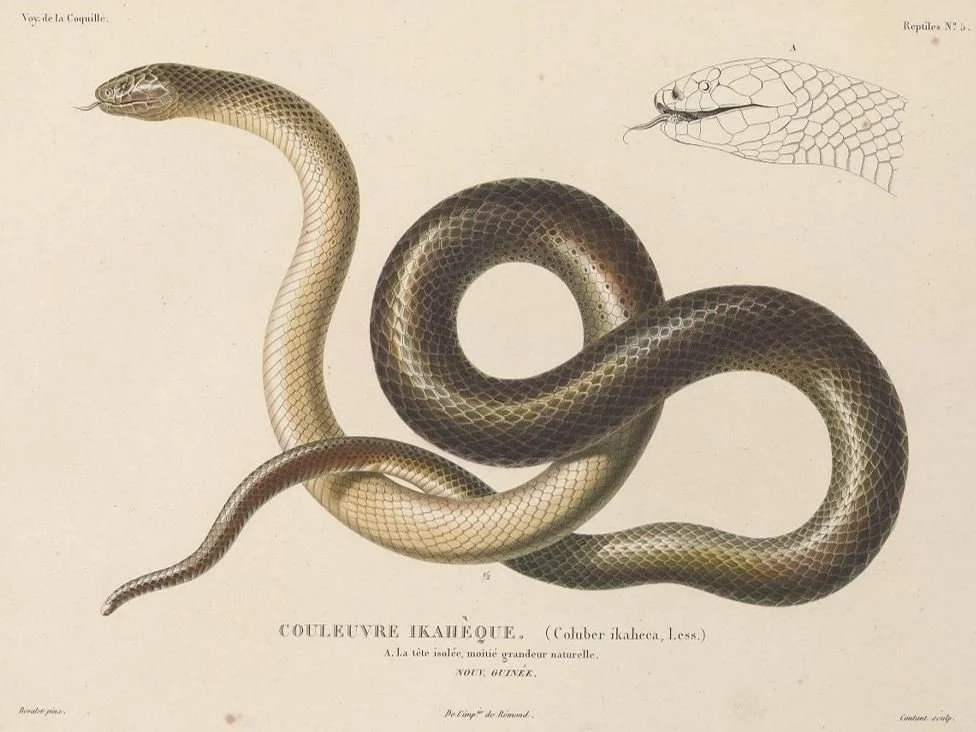
Some history and some natural history. Micropechis had its first outing in the scientific literature in 1830 when French surgeon, naturalist and zoologist René Primevère Lesson (1794-1849) described it as a new species of Coluber, initially using the spelling Coluber ikaheca. Lesson was describing specimens collected on his global voyage of 1822-1825 aboard the sailing ship La Coquille, and his visit to New Guinea and the nearby islands is best known for his observations of birds-of-paradise, for he was seemingly the first European scientist to see these birds alive in their natural habitat.
Caption: the original plate depicting this species, produced to accompany Lesson’s initial description of 1830. I haven’t translated the description’s text from the original French, but it looks like this particular specimen was plain in colour and markings relative to others.
The specific name for this snake (that is, the second part of its scientific binomial) – ‘Ikaheka’ – means ‘land eel’ in one of the local Papuan dialects, and this apparently refers to the fact that it’s sometimes associated with streams and other damp habitats (O’Shea 2011). After Lesson’s description, there existed disagreement on what sort of snake the Ikaheka really was, some herpetologists of the late 1800s realizing that its front-fanged condition made it no Coluber, but instead perhaps a viper or sort of cobra. In 1896, George Boulenger realized that it was unusual enough to warrant its own genus, and thus Micropechis was coined. It has been asserted that this must be pronounced ‘microp-echis’, and not ‘micro-pechis’ (Warrell et al. 1996).
Caption: live Micropechis, encountered in the field in Karkar Island, New Guinea, in 2010. One of the reasons that snakes (and other reptiles) have such clean, glossy scales most of the time is that their scales have a self-cleaning, dirt-shedding micro-ornamentation. Image: Wolfgang Wüster, used with permission.
Micropechis is smooth-scaled, light brown overall, and marked with transverse reddish bands. It’s secretive, nocturnal or crepuscular, and hunts for reptiles, frogs and mammals in cluttered rainforest and wetland habitats. O’Shea (2011) noted that it often hides in piles of discarded coconut husks, and that snakes including Candoia ground boas and other Micropechis individuals are among its prey. It’s oviparous. It’s also regarded as dangerous to humans and several fatalities are on record. Most recorded bites have involved local swelling, myalgia, systemic bleeding and the passing of dark urine (Warrell et al. 1996).
The other Micropechis. There’s a reason why I said that M. ikaheka is the only currently recognized member of its genus. This is because Boulenger named a second species – M. elapoides – in the above-mentioned text of 1896. This second species was from the Solomon Islands and had originally been included in the genus Hoplocephalus (today restricted to the Australian Broad-headed snake H. bungaroides and kin). Some later authors went further in terms of making this species an ally of M. ikaheka, even regarding it as a subspecies of M. ikaheka (Loveridge 1948).
Caption: a live Solomons coral snake Salomonelaps par photographed in the field in 2015. This photo was uploaded to (and taken from) iNaturalist, which I encourage people to use themselves (I do, albeit not as regularly as I should). Image: jqrichmond, CC BY-NC (original here).
However, the Solomon Islands snake differs notably from Micropechis proper in the anatomy of its teeth, palatine bones, scalation and hemipenes and – according to McDowell (1970) – appears more closely related to the Solomons coral snake Salomonelaps par* and the Australian bandy-bandy snakes (Vermicella). For those reasons, McDowell gave it its own genus – Loveridgelaps – in 1970, this name honouring British zoologist Arthur Loveridge, best known for his work on snakes and lizards. The species is sometimes called the Solomon or Solomons small-eyed snake and is even more obscure than the one we’re mostly looking at here. Micropechis and Salomonelaps both remain poorly studied, but the view that they’re distinct at the generic level has been followed in the post-McDowell literature.
* And yes, the name is indeed spelt ‘Salomonelaps’, not ‘Solomonelaps’. McDowell (1970) only said of this spelling that it referred to “the geographic region to which the genus is confined” (p. 146), so we must assume that he was referring to the historic ‘Islas Salomón’ term for the Solomon Islands.
Whence within Elapidae? Micropechis is certainly an elapid, possessing the proteroglyphous (front-fanged) anatomy of this group as well as the characteristic palatal and facial bone configuration, head scalation and much more. Within this large group, its anatomy, distribution and molecular traits show that it’s part of the clade that includes the Australasian taipans, tiger snakes and kin in additional to the viviparous or true sea snakes (as opposed to the laticaudine sea kraits, which are less specialized for marine life). This group is generally termed Hydrophiinae, though Sanders & Lee (2008) argued that Oyxuraninae might be used for it instead.
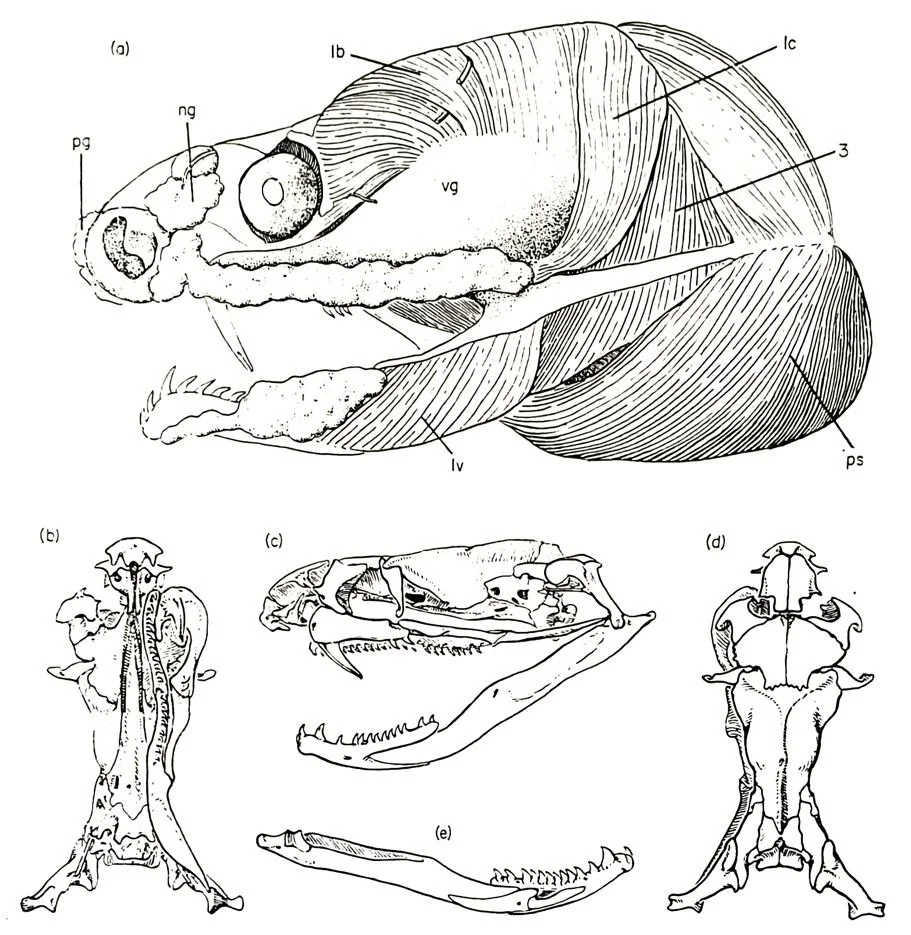
Caption: McDowell’s 1970 diagrams of a skinned head and skull of museum specimens of Micropechis in the collections of the American Museum of Natural History in New York. A few things are of interest. The venom gland (vg) is the large, convex area covering the cheek region; both the premaxillary salivary gland (pg) and nasal gland (ng) are visible on the snout. Image: McDowell (1970).
A diversion on palatine erection vs palatine dragging. In a moderately influential article of 1970, Samuel B. McDowell of Rutgers University in New Jersey argued that elapids possess two distinct ways of erecting their fangs. In one group – it includes all African, Asian and American elapids in addition to laticaudine sea snakes and Parapistocalamus of Bougainville Island – McDowell proposed that the vaguely triangular palatine bone is pulled vertically as the maxilla (the bone bearing the fang) is erected to deploy the fang. The palatine thus (so McDowell thought) ends up with a strong anterodorsal inclination during fang erection (meaning that it projects forwards and upwards at a diagonal slant). He termed the snakes that do this the ‘palatine erectors’ (McDowell 1970).
Caption: at left, from top to bottom, (A) the palatine and pterygoid bones (with the palatine, the more anterior of the two, at right) of a cobra, both in medial (inner) view, (B) the left palatine and anterior part of the pterygoid of a taipan in medial and (C) lateral view, and likewise for a death adder in (D) medial and (E) lateral views. The difference in shape of the cobra palatine from those of the taipan and death adder is obvious. At right, a ‘palatine erector’ (a cobra) at the top, and ‘palatine dragger’ (a taipan) below, these diagrams showing palatine movement as hypothesized by McDowell (1970). The palatine is the bone inboard of the fang-bearing maxilla (mx). It’s raising up into a diagonal position in the ‘erector’ and is pointing forwards at semi-horizontal orientation in the ‘dragger’. But be sure to check the main text! Images: Deuful & Cundall (2010).
Something different, McDowell proposed, happens in hydrophiines. In these animals, the palatine is bar-like, rather than subtriangular. As the maxilla is erected to deploy the fang, the palatine – McDowell thought – remains subhorizontal and in line with the similarly-shaped pterygoid to its posterior. Connection between the maxilla and palatine then meant that the latter is dragged anteriorly, such that its tip ended up being closer to the snout tip than is the leading edge of the maxilla (McDowell 1970). He termed these snakes the ‘palatine draggers’.
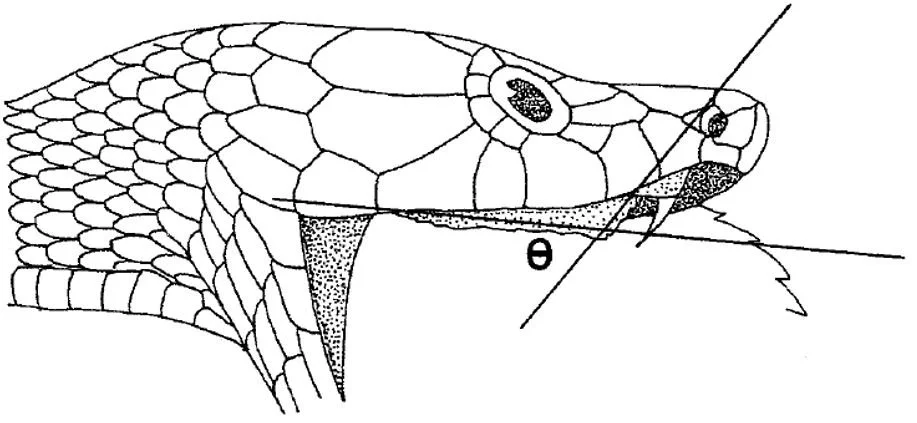
Caption: lest we forget, we’re actually here to talk about Micropechis. Here’s an especially useful, detailed shot of the face. The small, dark eyes are a familiar feature, but note also the slightly downturned snout (suitable for a snake that burrows in leaf litter, under logs and so on) and convex cheek regions (that are in keeping with moderately large venom glands). Image: Wolfgang Wüster, used with permission.
But here’s the problem. It turns out that the bones of live animals don’t behave or operate in the way you might think from museum specimens alone, dry skulls especially. McDowell didn’t precisely describe how he examined cranial kinesis in elapids, but it seems from the context of his work that he was manipulating skulls and/or skinned specimens, and not looking at the performance of live snakes. In a series of recent studies, Alexandra Deufel and David Cundall examined elapid skull function by filming live snakes as they feed, and then used these data to work out what’s happening with the skull bones (Deuful & Cundall 2003, 2006, 2010).
The bad news is that McDowell’s hypotheses of ‘palatine erection’ and ‘palatine dragging’ don’t really stand up, in part because the palatine and other bones don’t act as linked ‘pullers’ and ‘pushers’ of other bones. Instead, it’s the contractions of the protractor and levator pterygoidei muscles that are the main drivers of movement here: as the maxilla is protracted, the palatine moves anterolaterally (forwards and sideways). For McDowell’s ‘palatine erectors’, the palatine is indeed elevated as he proposed (Deuful & Cundall 2003), albeit not only because it’s attached to the maxilla as McDowell thought. In McDowell’s supposed ‘palatine draggers’, a strong ligamentous connection between the maxilla and palatine makes the palatine flex laterally (outwards) at its joint with the pterygoid, and ventrally (downwards) as the maxilla is protracted (Deuful & Cundall 2010). The palatine isn’t ‘dragged’ anteriorly as McDowell thought. Both ‘erectors’ and ‘draggers’ end up with a ventrally convex palatine-pterygoid bar during protraction.
Caption: diagram from Deuful & Cundall (2003), showing a ‘palatine erector’ elapid undergoing protraction of its right maxilla and palatine-pterygoid bar, the long axes of the palatine and pterygoid being shown via the intersecting lines. One thing that I haven’t discussed in this article is that the palatine rotates about its long axis. Hey, I can’t do everything. Image: Deuful & Cundall (2003).
What this means, ultimately, is that so-called palatine erectors and draggers don’t have the substantial differences in palatine movement that McDowell proposed. In any case, the palatine isn’t all that important in prey apprehension and swallowing, since it’s the pterygoid to its posterior that does the bulk of the work in ratcheting back and forth and ‘walking’ objects toward the throat. And if ‘erectors’ and ‘draggers’ aren’t performing the fundamentally different actions that McDowell thought they were, we really should give up on those terms (Deuful & Cundall 2010). I’ve continued to use them here because I’m discussing these hypotheses within a historical context.
But… come on, whence Micropechis? Anyway, the whole reason for this long discussion about palatine erectors and palatine draggers is to say that Micropechis has, historically, been considered an archaic member of the ‘palatine dragger’ assemblage. DNA-based studies that include Micropechis have appeared in print ever since the late 1990s, and they mostly find what was already suspected on the basis of anatomy: Micropechis is an early-diverging hydrophiine, outside the clade that includes Australasian taipans, tiger snakes and kin, and the viviparous or true sea snakes (Sanders & Lee 2008, Sanders et al. 2008, Pyron et al. 2013, Figueroa et al. 2016, Strickland et al. 2016).
Caption: highly simplified elapid cladogram (based mostly on Pyron et al. (2013)), shown to emphasize that Micropechis is closer to Australian viviparous elapids and true sea snakes than are sea kraits and Old World elapids like cobras. Images: Micrurus, public domain; Naja, kalmalnv, CC BY 3.0 (original here); Laticauda, Jens Petersen, CC BY-SA 3.0 (original here); Micropechis, Wolfgang Wüster (used with permission); Pseudechis, Smacdonald, CC BY 3.0 (original here); Oyxuranus, AllenMcC., CC BY-SA 3.0 (original here); Hydrophis Rasmussen et al. (2011), CC BY 2.5 (original here).
However, variations in results include the grouping of Micropechis with the whipsnake genus Demansia (Keogh 1998), and the recovery of both genera in a clade that also includes taipans (Oxyuranus), brown snakes (Pseudonaja) and black snakes (Pseudechis) (Scanlon & Lee 2004). Most recently, Micropechis has tended to group with whipsnakes and the New Guinean Toxicocalamus (Pyron et al. 2013, Figueroa et al. 2016). Incidentally, some Toxicocalamus species look superficially much like Micropechis, so much so that certain Toxicocalamus specimens were initially misidentified as individuals of Micropechis (O’Shea et al. 2018).
Caption: Toxicocalamus specimens can look quite similar to Micropechis. This montage, from O’Shea et al. (2018), shows museum specimens of (A, A') T. ernstmayri, (B, B') T. grandis and (C, C') a pale individual of Micropechis ikaheka. The colour coding of the head scales shows that Micropechis is similar to Toxicocalamus in having six supralabials (orange), one anterior temporal (yellow), and two posterior temporals (blue), but differs from it in possessing a temporolabial (red). Image: O’Shea et al. (2018).
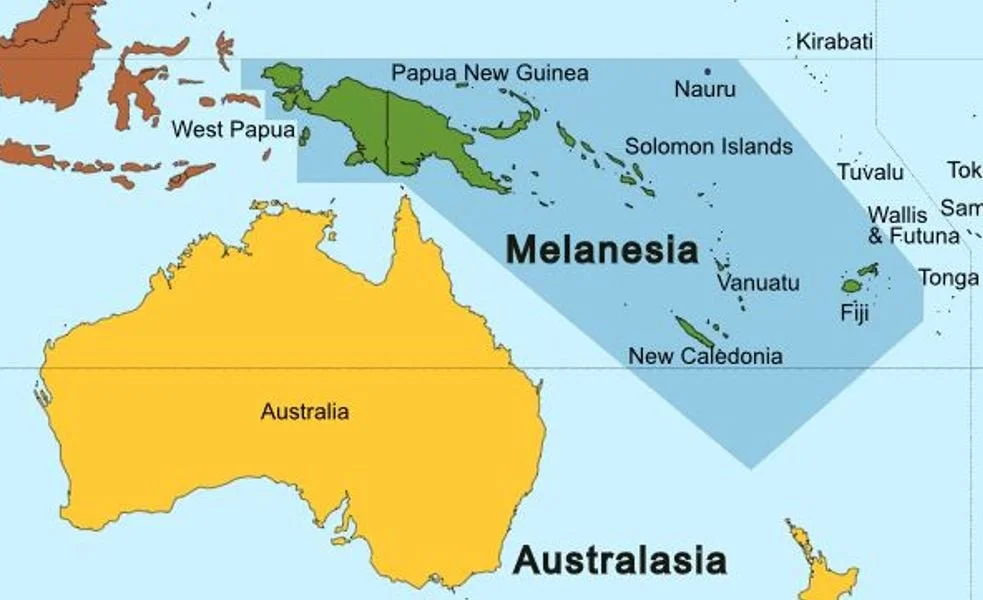
One thing worth emphasizing here is that several of the hydrophiines mentioned or discussed so far are not Australian like good, classic terrestrial hydrophiines, but are Melanesian: that is, they inhabit New Guinea, the Solomon Islands, Fiji and other islands of the region. That goes for the aforementioned Micropechis, Salomonelaps, Loveridgelaps and Toxicocalamus, but also for the Fiji snake Ogmodon vitianus and the New Guinean crowned snakes (Aspidomorphus). In addition, these are generally all cryptozoic: that is, they spend a lot of time in leaf litter, and under logs and rocks.
Caption: Melanesia – originally named on the basis of its dark-skinned people – includes New Guinea in the west, Fiji in the east, and also the Solomon Islands, New Caledonia, Vanuatu and Nauru. The region has a fascinating reptile fauna that includes unusual elapids, giant geckos, far-flung iguanians and remarkable skinks. Image: Oceania UN Geoscheme, CC BY 3.0 (original here).
What’s implied by the distribution and anatomy of these snakes is that they represent a sort of ‘ancestral grade’ for hydrophiines, and that the Australian terrestrial lineages and true sea snakes evolved from snakes of this sort. If this is correct, hydrophiines have their roots in the wet tropical forests of Melanesia, in cryptozoic snakes that are quite different ecologically from classic Australian hydrophiines (let alone sea snakes), and that they moved into Australia from the north. Here I want to mention in passing that the idea of hydrophiines – yes, all of them – originating from marine forms has been mentioned more than once.
At the moment, I think this this scenario (of Melanesian, cryptozoic ancestry) still appears broadly supported, though works finding whipsnakes and such to be within that ‘ancestral grade’ do suggest that things are more complex. Maybe there were multiple independent invasions of Australia, or maybe there was repeated exchange between Melanesia and Australia. Exactly that was promoted by Strickland et al. (2016). Also on the subject of hydrophiine evolution, molecular clock estimates indicate that the entire radiation is young, the divergence between Laticauda sea kraits and hydrophiines happening about 12 million years ago, in the middle of the Miocene. The 140 or so oviparous and viviparous terrestrial hydrophiines and 64 or so species of sea snake all emerged very rapidly between about 10 and 6 million years ago (Sanders et al. 2008), in the late Miocene. This is a geologically young, explosively successful radiation, and there’s a lot to say about it.
Caption: two different Micropechis specimens from Karkar Island off the north-east coast of Papua New Guinea, showing some of the variation in striping and colour present in this species. Warrell et al. (1996) noted that the name ‘tiger snake’ is used for the species in their study region. The animal at left was 1.5 m long; the one at right 1 m long. An especially darkly pigmented head is a common feature of terrestrial hydrophiines. Images: Warrell et al. (1996).
For now, we end here, and I’m pleased to have ‘rescued’ another Tet Zoo squamate-themed article from the archives. For previous Tet Zoo posts on snakes and other squamates see…
Cambodia: now with dibamids!, May 2011
The Tet Zoo Guide to Mastigures, August 2018
The Remarkable Basilisks, May 2023
Do Lizards Really Have ‘Mite Pockets’?, March 2024
Meeting Lake Zacapu’s Garter Snake, March 2024
Ray Hoser, Number 1 Taxonomic Vandal, May 2024
The Mysterious Dibamids, May 2024
The Rehabilitation of Günther’s Black Cameroonian Snake, May 2024
Refs – -
Deufel, A. & Cundall, D. 2003. Prey transport in “palatine-erecting” elapid snakes. Journal of Morphology 258, 358-375.
Deufel, A. & Cundall, D. 2006. Functional plasticity of the venom delivery system in snakes with a focus on the poststrike prey release behavior. Zoologischer Anzeiger 245, 249-267.
Deufel, A. & Cundall, D. 2010. Functional morphology of the palato-maxillary apparatus in “palatine dragging” snakes (Serpentes: Elapidae: Acanthophis, Oxyuranus). Journal of Morphology 271, 73-85.
Keogh, J. S. 1998. Molecular phylogeny of elapid snakes and a consideration of their biogeographic history. Biological Journal of the Linnean Society 63, 177-203.
Loveridge, A. 1948. New Guinean reptiles and amphibians in the Museum of Comparative Zoology and United States National Museum. Bulletin of the Museum Comparative Zoology, Harvard 101, 305-430.
McDowell, S. B. 1970. On the status and relationships of the Solomon Island elapid snakes. Journal of Zoology 161, 145-190.
O’Shea M. 2011. Venomous Snakes of the World. New Holland Publishers, London.
O’Shea, M., Herlihy, B., Paivu, B., Parker, F., Richards, S. J. & Kaiser, H. 2018. Rediscovery of the rare Star Mountains Worm-eating Snake, Toxicocalamus ernstmayri O’Shea et al., 2015 (Serpentes: Elapidae: Hydrophiinae) with the description of its coloration in life. Amphibian & Reptile Conservation 12, 27-34.
Scanlon, J. D. & Lee, M. S. Y. 2004. Phylogeny of Australasian venomous snakes (Colubroidea, Elapidae, Hydrophiinae) based on phenotypic and molecular evidence. Zoologica Scripta 33, 335-366.
Sanders, K. L. & Lee, M. S. Y. 2008. Molecular evidence for a rapid late-Miocene radiation of Australasian venomous snakes (Elapidae, Colubroidea). Molecular Phylogenetics and Evolution 46, 1165-1173.
Sanders, K. L., Lee, M. S. Y., Foster, R. & Keogh, J. S. 2008. Molecular phylogeny and divergence dates for Australasian elapids and sea snakes (hydrophiinae): evidence from seven genes for rapid evolutionary radiations. Journal of Evolutionary Biology 21, 682-695.
Strickland, J. L., Carter, S., Kraus, F. & Parkinson, C. L. 2016. Snake evolution in Melanesia: origin of the Hydrophiinae (Serpentes, Elapidae), and the evolutionary history of the enigmatic New Guinean elapid Toxicocalamus. Zoological Journal of the Linnean Society 178, 663-678.
Warrell, D. A., Hudson, B. J., Lalloo, D. G., Trevett, A. J., Whitehead, P., Bamler, P. R., Mamy Ranaivoson, Wiyono, A., Richie, T. L., Fryauff, D. J., O’Shea, M. T., Richards, A. M. & Theakston, R. D. G. 1996. The emerging syndrome of envenoming by the New Guinea small-eyed snake Micropechis ikaheka. Quarterly Journal of Medicine 89, 523-530.