For some years now, a prolific amateur herpetologist has published an absolutely extraordinary number of new taxonomic names for snakes, lizards and other reptiles…
Meeting Lake Zacapu’s Garter Snake
The Remarkable Basilisks
Way back in 2009 (the ScienceBlogs years) I published a Tetrapod Zoology article titled ‘Tell me something new about basilisks, puh-lease’ wherein I lamented the fact that people only ever say the same one thing about basilisks. Aware that there’s all too little squamate content here at Tetrapod Zoology ver 4, I here present a much augmented and updated version of that article…
Cases of Over-Enthusiastic Swallowing, Often (But Not Always) Ending in Death
Back in 2009 (at TetZoo ver 2, the ScienceBlogs version) I ran a series of articles on ‘over-enthusiastic swallowing’: on cases where carnivorous animals have died from choking. You see, carnivorous animals of many sorts often die from choking, and field biologists have done a good job of recording many such instances in the literature. In the interests of having this material available once more, rather than corrupted and only findable via the wayback machine, I’ve here gathered those articles together, and here they are again…
The Water Monitor Complex, an Introduction
Why the World Has to Ignore David Peters and ReptileEvolution.com
Corucia of the Solomon Islands, Most Amazing of Skinks
SKINKS! Again.
Caption: a captive Corucia in a commercial collection. Image: Darren Naish.
Skinks are an enormous, globally distributed group of lizards. As of December 2019, there are around 1685 recognised species, accounting for about 25% of living lizard diversity (there are about 6780 lizard species in total), and – perhaps unsurprisingly – I’ve written about them quite a lot at TetZoo… though it’s now hard to appreciate this, since the articles concerned have variously been vandalised, curtailed or paywalled by the hosters of TetZoo ver 2 and ver 3. See links below for the wayback machine versions of these articles.
Caption: there’s a lot about skinks in the Tet Zoo archives, please see the links below. Thank Christ for wayback machine.
Among the most remarkable and striking of skinks is the large Solomon Islands skink or Monkey-tailed skink Corucia zebrata, a prehensile-tailed, mostly green, arboreal skink, and the only member of its genus (though read on). Not only is this amazing lizard green, arboreal and equipped with a powerful prehensile tail, it’s also a giant, especially big specimens reaching 72 cm in total length. This makes it the biggest known skink. It first became known to science in 1856 when indefatigable taxonomist John E. Gray tersely described specimens brought to London by John MacGillivray after his voyage aboard the HMS Herald, the type specimens coming specifically from San Christoval (today termed San Cristobal or Makira) in the Solomon Islands (Gray 1856).
Caption: the Solomon Islands. Image by OCHA (original here), CC BY 3.0.
The lizards appear widespread throughout the archipelago (Makira is one of the most southerly islands there) and are variable, differing in eye colour, size, and in the configuration and size of their scales. Some experts think that subspecies should be named to reflect this variation, and the smaller, paler-eyed northern form was named C. z. alfredschmidti in 1997 (Köhler 1997). Maverick Australian bad boy herpetologist Raymond Hoser has claimed the existence of several entirely new species of Corucia, one of which he named for his mother. If you want to know more about Mr Hoser (and why he’s a total joke) see the TetZoo article here.
Caption: a captive Corucia in a private collection. Note the dark irides which make this individual look different from some of the other animals shown here. Image: S. Hilgers.
So far, all published work on the phylogeography and variation within Corucia finds it and its divergences to be young; as in, younger than about 4 million years old (Hagen et al. 2012). Yet it must have diverged from its closest living relatives 20 million years ago or more (we can infer this because fossils of other members of the same skink group are this old or older), meaning that the vast bulk of its lineages’ history remains completely unknown, for now.
Gray described Corucia as a new member of the ‘fish-scaled’ skink group. This seems a bit odd today, because we don’t refer to any skink by this moniker (to my knowledge). He evidently regarded it as part of the Australasian skink group that includes Egernia, Tiliqua (the blue-tongues) and kin though. Today we think (on the basis of molecular phylogenetics) that this is correct, and that Corucia is a lygosomine skink (Skinner et al. 2011, Pyron et al. 2013).
Caption: representatives of most (but not all) of the skink lineages currently regarded as ‘families’ by Hedges and colleagues. 1: Mabuya, of Mabuyidae. 2: Acontias, of Acontidae (I think it should really be Acontiidae). 3: Ristella, of Ristellidae. 4: Scincus, of Scincidae. 5: Lygosoma, of Lygosomidae. 6: Egernia, of Egerniidae. 7: Eugongylus, of Eugongylidae. These images are from my in-prep textbook, progress of which can be observed here. Images: Darren Naish.
Traditionally, all skinks are combined in the single family Scincidae. Most herpetologists argue that we should stick with this taxonomic system since there’s no dispute that Scincidae is a clade and thus no real need to shake things up. But some argue that putting all the species of this enormous, complex group into the same single ‘family’ obscures and under-emphasises its diversity and disparity and that it would be more realistic to split it into a whole bunch of families (nine in fact: Acontidae, Atechosauridae, Egerniidae, Eugongylidae, Lygosomidae, Mabuyidae, Ristellidae, Scincidae and Sphenomorphidae) (Hedges & Conn 2012, Hedges 2014). I’ve written about this situation before: see the articles below for more. If we follow this revised family-level classification, Corucia is part of Egerniidae.
Caption: substantially simplified cladogram depicting lygosomine skink phylogeny, mostly based on Pyron et al. (2013). Images (top to bottom): Wolfgang Wuster, H. Zell, $Mathe94$, Benjamint444 (all CC BY-SA 3.0), Mark Stevens (CC BY 2.0), W. A. Djatmiko, S. Caut et al. (both CC BY-SA 3.0).
The name Corucia is derived from ‘coruscus’ (meaning shimmering, and referring to the shiny scales), while zebrata is a reference to the stripes present in the specimens Gray was familiar with. Given that Solomon Islanders know this lizard and eat it, there was and is surely indigenous knowledge of the species and probably lore about it, though I haven’t encountered such so far. It’s generic name shouldn’t be confused with that of the Cretaceous fossil lizard Carusia, a possible relative of the living xenosaurids.
Here in the UK, it’s currently not difficult to encounter Corucia in captivity. I should add that it does well if conditions are right: as a canopy-dwelling lizard it needs tall branches with suitable retreats, and some collections (most notably the Philadelphia Zoo) have been breeding Corucia for over 40 years now. They’re not especially active during peak visitor time at zoos, mostly because they’re crepuscular. They’re also exclusively herbivorous and are in fact the only skinks said to be committed to a plant diet. Leaves, shoots, flowers and fruit are all consumed, including those of toxic species. Their dung has a distinctive aroma and it’s apparently possible to locate trees inhabited by this species by smell alone: Harmon (2002) used this technique, making his study “the first documented use of olfactory cues to locate skinks in the wild” (p. 177).
Caption: fine side-eye from this captive Corucia at Bristol Zoo, UK. Image: Darren Naish.
Corucia is viviparous with a 6 to 8 month gestation, but the big deal about its viviparous strategy is that its babies are proportionally enormous, being about half the size of the mother. They can be over 30 cm long and weigh 175 g. Unsurprisingly, only a single baby is normally produced, though rare cases of twins and triplets are on record.
Corucia is also a social skink. In this, it’s far from unique, since egerniids of more than 20 species live together in family groups and even exhibit monogamy, kin recognition, colonial living and co-operation. Juvenile Corucia sometimes stay with their parental group for an extended period and mothers are reported to be highly protective of newborn juveniles (Wright 2007), which is what theory predicts given the substantial material investment involved in growing such a large baby. Also interesting is that not all the adults which form social groups in this species appear related (Wright 1996), and that Corucia groups are even known to allow orphaned juveniles to join their groups (read on…). Some juveniles do apparently leave their parental group to join others (Wright 2007).
Caption: dark-eyed captive Corucia, and here’s proof that this arboreal lizard will - in captivity - drink from standing water (at least some arboreal lizards don’t do this, they rely only on water droplets on leaves). Image: S. Hilger.
Studies of wild-living Corucia on Ugi Island in the Solomon archipelago showed that individuals living less than 150 m apart were likely to be related, but also that individuals wandered for several kilometres (Hagen et al. 2013). Telemetry results obtained in an earlier study (Hagen 2011) indicate that this sort of dispersal is unusual, however, given that Corucia is mostly sedentary with home ranges being equivalent to the canopy of a single tree. Maybe this explains why groups are apparently happy to ‘adopt’ lone youngsters – they may well be related to the members of the group already. After all, we know that kin selection is at play elsewhere in social egerniids.
One of the latest papers discussing social behaviour in these skinks is also among the most shocking, since it reports the occurrence of a Corucia group living together in a deep tree hole, and one that was flooded at its bottom. Remarkably, some of the Corucia in the hole were fully submerged and located beneath the water surface at the time of discovery. To my supreme frustration, I can’t locate this publication right now, even though I recall downloading it (it was a short note in, perhaps, Salamandra or Journal of Herpetology). Let me know if you know the paper concerned. It was such a bizarre report that more information is needed. And I guarantee that it’s legit and that I didn’t dream it.
Caption: a captive Corucia at Bristol Zoo. Note the sharply curved claws and interesting nose in these lizards. Image: Darren Naish.
Finally, what does the future hold for this amazing lizard? Unsustainable destruction of forests on the Solomon Islands poses a problem, as does local hunting for the pot and collecting for the pet trade: between 1992 and 1995, 12000 animals were exported for this reason, mostly to the USA (Mann & Meek 2004). Consequently, Corucia is now being considered for inclusion on Appendix I of CITES, with captive breeding likely being crucial to its persistence.
Caption: another captive Corucia. This image is useful and interesting because it shows the cross-sectional shape of the body: note that the side of the body is flat and that there’s an obvious change in angle between the side and dorsal surface. Image: TimVickers (original here), public domain.
A giant, fully herbivorous, slow-breeding, social skink is such a special animal that we must make effort to ensure its survival into the future. And that’s where we must end.
For previous TetZoo articles on skinks, see…
Mystery emo skinks of Tonga!, October 2010
Isopachys: worm-like skinks from Thailand and Myanmar, November 2010
Hammer-toothed skink SMASH!, November 2012
Skinks, Skinks, Skinks!, October 2014
Terror skinks, social skinks, crocodile skinks, monkey-tailed skinks… it's about skinks (skinks part II), October 2014
Sandfishes and kin: of sand-swimming, placentation, and limb and digit reduction (skinks part III), November 2014
The Madagascan Skink Amphiglossus Eats Crabs, February 2016
Refs - -
Gray, J. E. 1856. New genus of fish-scaled lizards (Scissosarae) from New Guinea. Annals and Magazine of Natural History (2) 18: 345-346.
Hagen, I. J. 2011. Home ranges in the trees: radiotelemetry of the Prehensile tailed skink, Corucia zebrata. Journal of Herpetology 45, 36-39.
Hagen, I. J., Donnellan, S. C. & Bull, C. M. 2012. Phylogeography of the prehensile-tailed skink Corucia zebrata on the Solomon Archipelago. Ecology and Evolution 2, 1220-1234.
Hagen, I. J., Herfindal, I., Donnellan, S. C. & Bull, C. M. 2013. Fine scale genetic structure in a population of the prehensile tailed skink, Corucia zebrata. Journal of Herpetology 47, 308-313.
Harmon, L. J. 2002. Some observations of the natural history of the Prehensile-tailed skink, Corucia zebrata, in the Solomon Islands. Herpetological Review 33, 177-179.
Hedges, S. B. 2014. The high-level classification of skinks (Reptilia, Squamata, Scincomorpha). Zootaxa 3765, 317-338.
Hedges, S. B . & Conn, C. E. 2012. A new skink fauna from Caribbean islands (Squamata, Mabuyidae, Mabuyinae). Zootaxa 3288, 1-244.
Köhler, G. 1997. Eine neue Unterart des Wickelschwanzskinkes Corucia zebrata von Bougainvillle, Papua-Neuguinea. Salamandra 33 (1), 61-68.
Mann, S. L. & Meek, R. 2004. Understanding the relationship between body temperatureand activity patterns in the giant Solomon Island skink, Corucia zebrata, as a contribution to the effectiveness of captive breeding programmes. Applied Herpetology 1, 287-298.
Pyron, R. A., Burbrink, F. T. & Wiens, J. J. 2013. A phylogeny and revised classification of Squamata, including 4161 species of lizards and snakes. BMC Evolutionary Biology 2013, 13:93.
Skinner, A., Hugall, A. F. & Hutchinson, M. N. 2011. Lygosomine phylogeny and the origins of Australian scincid lizards. Journal of Biogeography 38, 1044-1058.
Wright, K. 1996. The Solomon Islands skink. Reptile & Amphibian Magazine 3 (2), 10-19.
Wright, K. M. 2007. Captivating giants. Reptiles Magazine 15 (12), 54-68.
The Mostly Arboreal African Gastropholis Lizards
TetZoo hasn’t had nearly enough lizards lately. If you’re a regular reader you might recall the slow-burn series on LACERTIDS, the Old World group that are the ‘typical’ lizards of western and central Europe and hence the first group to become known to European scientists.
Caption: if you’re European, lacertids are the lizards you know best. In the UK, we only have two natives, one of which is Zootoca vivipara, the Viviparous lizard. Here’s a wild individual. Image: Darren Naish.
European lacertids – thinking here of Zootoca (viviparous lizards), Lacerta (sand lizards, green lizards and kin) and Podarcis (wall lizards) in particular – are quite well known and it’s easy to find information on them; if, that is, you’ve combed through books on lizards in quest of hot lacertid info. But there are a whole bunch of African taxa within Lacertidae: what’s the deal with them, and why don’t we hear more about them? Well, comparatively little is known about them, good photos of them aren’t that abundant, and they’re rare in museum collections (Arnold 2004).
Caption: a captive Green keel-bellied lizard Gastropholis prasina, in repose. Check out that tail! Images: Darren Naish.
I recently got to see a live lacertid of a type I’d never seen before: a Green keel-bellied lizard Gastropholis prasina, a shockingly long-tailed, green, east African lacertid endemic to the coastal forests of Mozambique, Tanzania and Kenya. It reaches 40 cm in total (which seems pretty huge for a lacertid), but is more usually 25-35 cm. Most of this is formed by the slender, prehensile tail. These are diurnal lizards, and one of several things that makes them unusual among lacertids is that they’re arboreal, and do just about everything up in the trees. They sleep there, fight there, mate there and lay their eggs there (in damp hollows). Individuals of G. prasina have been found 12 metres up in trees (Spawls et al. 2018).
Caption: an 1886 illustration of the Striped keel-bellied lizard G. vittata, from J. G. Fischer’s publication. Image: J. G. Fischer, public domain.
Well…. the Green keel-bellied lizard lives this way, anyway. Animals refuse to be constrained by anatomy and not all members of this genus are arboreal. The Striped keel-bellied lizard G. vittata is apparently terrestrial despite being proportionally pretty much exactly like G. prasina and being equipped with a really long, prehensile tail (Spawls et al. 2018). What this most likely shows is that G. vittata has only recently transitioned to terrestrial life, and that its anatomy still harks back to an arboreal ancestry.
Caption: you can see why this species is called Gastropholis echinata (the species name meaning ‘spiny’). It’s from a 1919 description of the species by K. P. Schmidt. Image: K. P. Schmidt/AMNH, public domain.
Two other species are presently included in Gastropholis. G. echinata occurs throughout those countries bordering the Gulf of Guinea while G. tropidopholis is endemic to the Democratic Republic of the Congo.
Caption: another Gastropholis species: the Congolese G. tropidopholis. Again this image is from a 1919 paper by K. P. Schmidt. Image: K. P. Schmidt/AMNH, public domain.
Convergence with grass lizards…. and monitors? As implied by the name, one of the unusual things about these lizards is that their ventral scales are keeled. That’s pretty odd, since lizards (and other reptiles) ‘ordinarily’ have smooth ventral scales. Keeled ventral scales are also present in at least some species of Adolfus – another climbing African lacertid – and also in Takydromus, the east Asian grass lizards. Like Gastropholis, Takydromus has a strikingly long tail which it uses in climbing (albeit in grasses rather than high in trees).
These lizards also (together with Philochortus, another poorly known African lacertid) share especially tall, blade-like neural spines on some of their caudal vertebrae (Arnold 1997, 2004). Neither Takydromus nor Philochortus are at all close to Gastropholis in lacertid phylogeny, so any similarities have to be a product of convergence (Arnold 2004). If you want to learn more about Takydromus, I wrote about it here at ver 2, back in 2013.
Caption: a Takydromus - an Asian grass lizard - in captivity. These lacertids are similar to the African Gastropholis species in several respects, but are not that closely related to them. Image: Darren Naish.
On the subject of convergence, I can’t help but also regard these mostly arboreal, often green lacertids as convergent with the also long-tailed, also green prasinoid tree monitors of northern Australasia. Prasinoids – the Green tree monitor Varanus prasinus and its several close relatives – are larger than Gastropholis species (40 cm vs 60-110 cm, total length) and have several varanid-flavoured features lacking in lacertids (a long forked tongue, blade-like teeth, longer and more dextrous digits and – probably – enhanced cognition). Prasinoids seem more formidable as predators than is Gastropholis, since they predate on small mammals, big spiny insects and so on. This could just be a consequence of size though, and Gastropholis is reported to predate on smaller lizards so might well be quite formidable too (Spawls et al. 2018). If you’re wondering why there are both Varanus and Gastropholis taxa within the species name prasinus (or prasina), it’s because this means ‘light green’.
Caption: I’ve had reason to draw prasinoids - green tree monitors - on several occasions, and here’s a montage depicting several species. Can these varanids be considered convergent with the long-tailed, arboreal lacertid Gastropholis? Hmm, maybe. Images: Darren Naish.
I’ve written about prasinoids at TetZoo: please go here (reminder that I now link to wayback machine versions of my ver 3 articles, since SciAm has either mutilated my articles or paywalled them).
Where in the family tree? Where do the Gastropholis species fit within the lacertid radiation? None of these lizards are well known and they tend to be missed entirely from popular works that mention or discuss lacertids, this creating the impression that Lacertidae doesn’t really have a presence in the African tropics, which it totally does. Within recent decades it’s been widely agreed that these lizards are part of the equatorial lacertid clade that includes Adolfus and kin. This whole group is probably part of Eremiadini, the lacertid clade that also includes the fringe-toed lizards (Acanthodactylus) and racerunners (Eremias) (e.g., Peréz I de Lanuza & Font 2014)… though be sure to see the TetZoo article on racerunners and kin to get some perspective on the different phylogenetic proposals that have been put forwards for these animals.
Caption: a substantially simplified lacertid phylogeny, showing the approximate structure pieced together in assorted studies. Gastropholis by Darren Naish, Acanthodactylus by Richard Hing, Eremias by Yuriy75 (CC BY-SA 3.0; original here), Takydromus by Acapella (CC BY-SA 3.0; original here), Lacerta by Darren Naish, Gallotia by Petermann (CC BY-SA 3.0; original here), Psammodromus by Wolfgang Wüster. Image CC BY-SA.
Arnold (1989) examined anatomical features and found Gastropholis to form a clade with Bedriagaia, Holaspis and Adolfus, the four forming what he termed ‘the Equatorial African clade’. I have to mention in passing that Holaspis is especially interesting because it’s able to glide and has seemingly evolved this ability as an exaptation (Arnold 2002). That’s a story for another time though. Greenbaum et al. (2011), using data from several genes, found Gastropholis to be the sister-group to Adolfus sensu stricto (Adolfus of tradition is paraphyletic with respect to Holaspis, so some of its populations are now separated and in the 2011 genus Congolacerta).
A note on the future. What else do we know about these lizards? They’re suspected to be in decline, given that the forests they inhabit are being destroyed, but hints that they might be adaptable enough to persist come from the fact that they’ve moved into cash crop plantations in some places (Spawls et al. 2018). The individual shown in my photos above and below is a captive and part of the pet trade. The trade in exotic reptiles (and amphibians) is mostly evil, exploitative and the opposite of anything that can be considered sustainable and beneficial. Having said that, the fact that populations of animals endangered in the wild are now being bred in captivity might provide some kind of safeguard for the future. That’s where we’ll end for now, but we’ll return to lacertids - and other lizards - sometime in the near future.
Caption: Green keel-bellied lizard in profile. Image: Darren Naish.
For previous TetZoo articles on lacertids (linking here to wayback machine versions to avoid issues with the hosting sites, SciAm in particular), see…
The New Forest Reptile Centre, August 2012
Tale of the Takydromus, September 2013
Racerunner Lizards of the World Unite, October 2014
Refs - -
Arnold, E. N. 1997. Interrelationships and evolution of the east Asian grass lizards, Takydromus (Squamata: Lacertidae). Zoological Journal of the Linnean Society 119, 267-296.
Arnold, E. N. 1989. Towards a phylogeny and biogeography of the Lacertidae: relationships within an Old-World family of lizards derived from morphology. Bulletin of British Museum of Natural History (Zoology) 55, 209-257.
Arnold, E. N. 2002. Holaspis, a lizard that glided by accident: mosaics of cooption and adaptation in a tropical forest lacertid (Reptilia, Lacertidae). Bulletin of British Museum of Natural History (Zoology) 68, 155-163.
Arnold, E. N. 2004. Overview of morphological evolution and radiation in the Lacertidae. In Pérez-Mellado, V., Riera, N. & Perera, A. (eds) The Biology of Lacertid Lizards. Evolutionary and Ecological Perspectives. Institut Menorquí d’Estudis. Recerca 8, 11-36.
Greenbaum, E., Villanueva, C. O., Kusamba, C., Aristote, M. M. & Branch, W. R. 2011. A molecular phylogeny of equatorial African Lacertidae, with the description of a new genus and species from the Democratic Republic of the Congo. Zoological Journal of the Linnean Society 163, 913-942.
Peréz I de Lanuza, G. & Font, E. 2014. Ultraviolet vision in lacertid lizards: evidence from retinal structure, eye transmittance, SWS1 visual pigment genes and behaviour. The Journal of Experimental Biology 217, 2899-2909.
The Most Amazing TetZoo-Themed Discoveries of 2018
As we hurtle toward the end of the year – always a scary thing because you realise how much you didn’t get done in the year that’s passed – it’s time to look back at just a little of what happened in 2018...
Caption: animals we will meet below, a montage. Images: (c) Philippe Verbelen, (c) Kristen Grace, Florida Museum of Natural History, Graham et al. (2018), CC BY-SA 4.0.
This article is not anything like a TetZoo review of 2018 (I’ll aim to produce something along those lines in early 2019), but, rather, a quick look at some of the year’s neatest and most exciting zoological (well, tetrapodological) discoveries. As per usual, I intended to write a whole lot more – there are so many things worthy of coverage – and what we have here is very much an abridged version of what I planned.
Thanks as always to those supporting me at patreon. Time is the great constraint (and finance, of course), and the more support I have, the more time I can spend on producing blog content. Anyway, to business…
The Rote leaf warbler. New passerine bird species are still discovered on a fairly regular basis; in fact three were named in 2018*. One of these is especially remarkable. It’s a leaf warbler, or phylloscopid, endemic to Rote in the Lesser Sundas, and like most members of the group is a canopy-dwelling, insectivorous, greenish bird that gleans for prey among foliage. Leaf warblers are generally samey in profile and bill shape, so the big deal about the new Rote species – the Rote leaf warbler Phylloscopus rotiensis – is that its bill is proportionally long and curved, giving it a unique look within the group. It superficially recalls a tailorbird. Indeed, I think it’s likely that the species would be considered ‘distinct enough’ for its own genus if there weren’t compelling molecular data that nests it deeply within Phylloscopus (Ng et al. 2018).
* The others are the Cordillera Azul antbird Myrmoderus eowilsoni and the Western square-tailed drongo Dicrurus occidentalis.
Caption: a Common chiffchaff Phylloscopus collybita encountered in western Europe, a familiar Eurasian-African phylloscopid leaf warbler. Image: Darren Naish.
The story of the Rote leaf warbler’s discovery is interesting in that it’s yet another recently discovered species whose existence and novelty was suspected for a while. Colin Trainor reported leaf warblers on Rote in 2004 but never got a good look at them, Philippe Verbelen observed them in 2009 and realised how anatomically unusual they were, and it wasn’t until 2015 that a holotype specimen was procured (Ng et al. 2018). I’ve mentioned before the fact that documenting and eventually publishing a new species is rarely an instant see it catch it publish it event, but a drawn-out one that can take decades, and here we are again. Also worth noting is that the existence of a leaf warbler on Rote was not predicted based on our prior knowledge of leaf warbler distribution in view of the deep marine channel separating Rote from Timor and lack of any prior terrestrial connection. Yeah, birds can fly, but members of many groups prefer not to cross deep water channels. In this case, this did, however, happen and most likely at some point late in the Pliocene (Ng et al. 2018).
Caption: Rote leaf warbler in life, a novel member of an otherwise conservative group. Image: (c) Philippe Verbelen.
Rote has yielded other new passerines in recent years – the Rote myzomela Myzomela irianawidodoae (a honeyeater) was named in 2017 – and it’s possible that one or two others might still await discovery there.
Neanderthal cave art. Hominins don’t get covered much at TetZoo, which is weird given the amazing pace of relevant recent discoveries and the fact that they’re totally part of the remit. I mostly don’t cover them because I feel they’re sufficiently written about elsewhere in the science blogging universe, plus I tend to be preoccupied with other things. Nevertheless, I take notice, and of the many very interesting things published in 2018 was Hoffman et al.’s (2018) announcement of several different pieces of Spanish rock art, seemingly made by Neanderthals Homo neanderthalensis. The art concerned involves hand stencils, abstract lines, squares and amorphous patches of pigment, always marked in red.
Caption: red abstract markings, discovered in several Spanish caves, are old, and in fact were seemingly made by hominins long before H. sapiens moved into Europe. The red sinuous marking and system of squares and lines near the middle of this photo are purported to have been made by Neanderthals (other images, depicting animals and present adjacent to these markings, were seemingly created more recently by H. sapiens individuals). Image: (c) P. Saura.
The main reason for the attribution of this art to Neanderthals is its age: uranium-thorium dating shows that it’s older than 64ka, which therefore makes it more than 20ka older than the time at which H. sapiens arrived in Europe (Hoffman et al. 2018). That seems compelling, and it’s consistent with a building quantity of evidence for Neanderthal cultural complexity which involves the use of shells, pigments, broken stalagmites and so on.
Caption: one of the most famous pieces of claimed Neanderthal rock art: the Gorham's Cave ‘hashtag’ from Gibraltar. Image: (c) Stewart Finlayson.
I should add here, however, that I’m slightly sceptical of the use of age as a guide to species-level identification. Why? Well, we have evidence from elsewhere in the fossil record that the range of a hominin species can be extended by around 100ka without serious issue (witness the 2017 announcement of H. sapiens remains from north Africa; a discovery which substantially increased the longevity of our species). In view of this, would a 20ka extension of H. sapiens’ presence in Europe be absolutely out of the question? Such a possibility is not supported by evidence yet, and I don’t mean to appear at all biased against Neanderthals.
A tiny Cretaceous anguimorph in amber, and other Mesozoic amber animals. As you’ll know if you follow fossil-themed news, recent years have seen the discovery of an impressive number of vertebrate fossils in Cretaceous amber, virtually all of which are from Myanmar and date to around 99 million years old. They include tiny enantiornithine birds, various feathers (most recently racquet-like ‘rachis dominated feathers’), the tiny snake Xiaophis, early members of the gecko and chameleon lineages and the small frog Electrorana. Many of these finds were published in 2018 and any one could count as an ‘amazing’ discovery.
Caption: the Barlochersaurus winhtini holotype, from Daza et al. (2018).
However, there’s one fossil in particular that I find ‘amazing’, and it hasn’t received all that much coverage. It’s the tiny (SVL* 19.1 mm!), slim-bodied anguimorph Barlochersaurus winhtini, named for a single, near-complete specimen subjected to CT-scanning (Daza et al. 2018). Remarkable images of its anatomical details are included in Daza et al.’s (2018) paper. It has short limbs, pentadactyl hands and feet and a slim, shallow, bullet-shaped skull. Phylogenetic study finds it to be somewhere close to, or within, Platynota (the clade that includes gila monsters and kin, and monitors and kin), or perhaps a shinisaurian (Daza et al. 2018). It could be a specialised dwarf form, or somehow more reflective of the ancestral bauplan for these anguimorph groups. Either way, it’s exciting and interesting. What next from Burmese amber?
* snout to vent length
Caption: Barlochersaurus in life. It’s about the size of a paperclip. Image: (c) Kristen Grace, Florida Museum of Natural History (original here).
The Reticulated Siren. Sirens are very special, long-bodied aquatic salamanders with reduced limbs and bushy external gills. They’re very weird. They can reach 95 cm in length (and some fossil species were even larger), lack hindlimbs and a pelvis, have a horny beak and pavements of crushing teeth, and eat plants in addition to gastropods, bivalves and other animal prey. A longish article on siren biology and evolution can be found here at TetZoo ver 3.
Caption: a life reconstruction of the Cretaceous siren Habrosaurus, showing features typical of the group. This animal could reach 1.5 m in total length. Image: Darren Naish (prepared for my in-prep texbook The Vertebrate Fossil Record, on which go here).
Until recently, just four living siren species were recognised. But it turns out that indications of a fifth – endemic to southern Alabama and the Florida panhandle – have been around since 1970 at least. Furthermore, they pertain to a big species, similar in size to the Great siren Siren lacertina. Known locally as the ‘leopard eel’ (a less than ideal moniker, given that there’s a real eel that already goes by this name), this animal has been published by Sean Graham and colleagues in the open-access journal PLoS ONE (Graham et al. 2018) wherein it’s formally christened the Reticulated siren S. reticulata. It reaches 60 cm in total length, has dark spots across its dorsal surface and a proportionally smaller head and longer tail than other Siren species.
A museum specimen of the species has been known since 1970 when its finder noted that it did “not conform” to descriptions of known species, and live specimens were collected by David Steen and colleagues in 2009 and 2014. Again, note that discovery and recognition was a drawn-out process. The discovery has, quite rightly, received a substantial amount of media coverage, and many interesting articles about the find are already online. Many of you will already know of David Steen due to his social media presence and Alongside Wild charity (which I’m proud to say I support via pledges at patreon).
Caption: the Reticulated siren paratype specimen, as described by Graham et al. (2018). Image: Graham et al. (2018), CC BY-SA 4.0. Original here.
The idea that a new living amphibian species 60 cm long might be discovered anew in North America in 2018 is pretty radical. I’m reminded of the 2009 TetZoo ver 2 article ‘The USA is still yielding lots of new extant tetrapod species’ (which is less fun to look at than it should be, since images aren’t currently showing at ver 2). Furthermore, Graham et al. (2018) discovered during their molecular phylogenetic work that some other siren species are not monophyletic but likely species complexes, in which case taxonomic revision is required and more new species will probably be named down the line.
And that’s where I must end things, even though there are easily another ten discoveries I’d like to write about. This is very likely the last article I’ll have time to deal with before Christmas. As I write, I’m preparing to leave for the Popularising Palaeontology conference which happens in London this week (more info here), and then there are Christmas parties and a ton of consultancy jobs to get done before the New Year. On that note, I’ll sign off with a festive message, as is tradition. Best wishes for the season, and here’s to a fruitful and action-packed 2019. Special thanks once again to those helping me out at patreon.
For previous TetZoo articles relevant to various of the subjects covered here, see…
The USA is still yielding lots of new extant tetrapod species, July 2009
THE AMAZING WORLD OF SALAMANDERS, October 2013
Chiffchaffs: a view of passerines from the peripheries (part I), August 2014
The Biology of Sirens, June 2016
Refs - -
Daza, J. D., Bauer, A. M., Stanley, E. L., Bolet, A., Dickson, B. & Losos, J. B. 2018. An enigmatic miniaturized and attenuate whole lizard from the mid-Cretaceous amber of Myanmar. Breviora 563, 1-18.
Hoffman, D. L., Standish, C. D., García-Diez, M., Pettitt, P. B., Milton, J. A., Zilhão, J., Alcolea-González, J. J., Cantelejo-Duarte, P., Collado, H., de Balbín, R., Lorblanchet, M., Ramos-Muñoz, J., Weniger, G.-Ch. & Pike, A. W. G. 2018. U-Th dating of carbonate crusts reveals Neandertal origin of Iberian cave art. Science 359, 912-915.
The Tet Zoo Guide to Mastigures
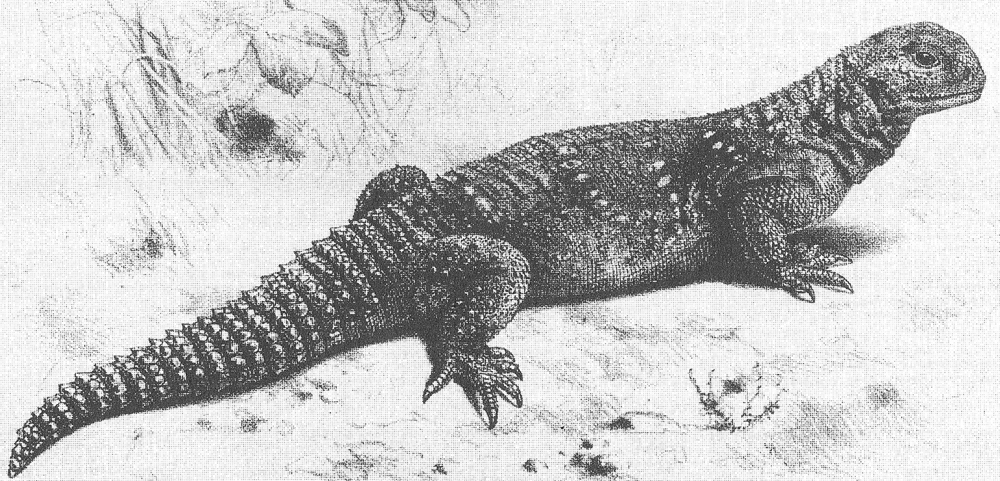
Among my favourite lizards are the Uromastyx agamids, variously termed mastigures, dabbs, dabs, dhubs, spinytails, spiny-tailed agamas, spiny-tailed lizards or thorny-tailed lizards. In the pet trade they’re often called ‘uros’. Here, I’ll be calling them mastigures.
Caption: a large mastigure is a fine, handsome, happy-looking lizard. The dark overall colour and yellow dorsal occellations show that this captive specimen is a Moroccan mastigure Uromastyx acanthinurus. Image: Darren Naish.
Mid-sized for lizards (25 cm in total length is typical, though read on), they’re rather chunky, short-headed and wide-bodied with a proportionally short, broad tail that’s covered in 10 to 30 transverse, parallel rows of posterodistally projecting spines. The rows have a ring-like form and (rather confusingly) are typically called whorls. The tail is said to function as a ‘burrow blocker’ and also to be lashed from side to side when deterring would-be attackers. Enlarged, thorn-like scales are also present on the hindlimbs of some species. The head is short and deep by lizard standards and a neat feature is that the labial scales are large, serrated structures that sometimes look like external pseudoteeth.
Caption: head detail of a captive U. acanthinurus. Note the pseudoteeth-like upper labial scales. The white exudate around the nostrils is pretty typical: it's salt discharge and evidence of nasal salt excretion typical for desert-dwelling lizards. Image: Darren Naish.
Mastigures are extremely variable in colour, ranging from almost black to almost white dorsally; areas of yellow and even bright orange are present in some species, sometimes forming eye-like markings, distinct spots large or small, or transverse bands. The head may be much darker than the rest of the animal, and sometimes the tail is different in colouration too. Adding to this complexity is that individuals change colour according to temperature and time of day. The tail is variable in size: it's similar in length to the body in most species but is very short and broad in a few species, most notably the Omani spiny-tailed lizard or Thomas's mastigure U. thomasi.
Caption: a captive U. thomasi. The complex colouration - the facial banding in particular - is notable, as is the very short, broad, plump tail. This is a small mastigure with a total length of less than 15 cm. Recent surveys indicate that it is now extinct on mainland Oman - its type location - and is now unique to Masirah Island where local extinction has also occurred due to habitat destruction. There are anecdotal 2012 references to its persistence on the mainland, however. Image: Darren Naish.
The teeth are especially interesting: they’re short, low-crowned and fused to the jaw bones on their lingual (tongue) side, are largest at the back of the jaws, have crescentic shearing tips, and possess oblique wear facets that become so pronounced with age that entire teeth can be worn right down to the jaw (Cooper & Poole 1973). As you might guess, these animals do not possess regular tooth replacement of the sort we associate with reptiles (Robinson 1976). This is linked with a style of jaw movement (termed propaliny) where the lower jaw slides forwards to create a shearing bite when the jaws are closed (Throckmorton 1976). In the premaxillae, the upper central incisiforms are replaced by projecting structures that have been interpreted as bony pseudoteeth (Anderson 1999), though I don’t know if the histological work required to demonstrate this has been performed and they might be fused teeth.
Caption: the skull of U. aegyptia, as scanned for The Deep Scaly Project and available here. Note that the partially fused teeth are largest posteriorly. The mandible is deep, the front of the dentary is toothless and bony pseudoteeth are present in the premaxilla. Image: Digimorph.
Mastigures occur throughout the steppes, deserts and semi-deserts of northern Africa, the Middle East and western and central Asia. They aren’t associated with dune-fields, instead inhabiting rocky or gravel-covered regions or areas with compacted sand. They use and build burrows that are sometimes 3 m long or so, though I would expect based on data from other burrow-digging reptiles that burrows at least twice as long might exist. ‘Colonial burrows’ have been mentioned in the literature (Anderson 1999), though I don’t know if this means that many burrows were located in close proximity or if the burrows were known to contain some or many lizards.
Caption: an Iraqi, Mesopotamian or Small-scaled spiny-tailed lizard Saara loricata (formerly U. loricatus), a mid-sized species of Iran and Iraq, as illustrated in one of Boulenger's 1885 catalogues of amphibians and reptiles kept in the collections of the British Museum. Image: Boulenger 1885.
Around 15 extant species are recognised within Uromastyx, five of which have been named since 1990: U. maliensis Joger & Lambert, 1996, U. occidentalis Mateo et al., 1999 (or 1998), U. leptieni Wilms & Böhme, 2001 (or 2000…), U. alfredschmidti Wilms & Böhme, 2001 (or 2000…) and U. yemenensis Wilms & Schmitz, 2007. The total number of recognised species is a bit vague since some taxa are regarded as subspecies by some authors and as distinct species by others. An additional three Asian species have recently been removed from Uromastyx and placed in the resurrected genus Saara, first named by Gray in 1845 (Wilms et al. 2009). Saara species possess so-called intercalary scales between the spine whorls on the tail and molecular data finds them to be the sister-group to Uromastyx (Tamar et al. 2018).
Caption: Persian or Iranian spiny-tailed lizard Saara asmussi, as illustrated in William Blanford's paper of 1876. This species occurs in Iran, southern Afghanistan and Pakistan. The Saara species were included within Uromastyx prior to Wilms et al. (2009). Image: Blanford 1876.
In recent years, Uromastyx mastigures have become increasingly common in the pet trade and it’s now normal to see them on show in places that sell pet reptiles. I have seen them in the wild while on fieldwork in the Sahara, but the individuals concerned were dead and I never have seen a live one in the wild.
Caption: a sadly deceased baby mastigure (probably U. acanthinurus), discovered in the Moroccan Sahara. Cause of death unknown. Note that the tail is fully developed and sports the full complement of tail spines, despite the animal's small size. Image: Darren Naish.
Biology and behaviour. Mastigures are omnivorous, but they’re (seemingly) essentially herbivorous as adults, only occasionally eating arthropods or smaller lizards. The presence of symbiotic gut flora has been demonstrated for some species (a feature seen elsewhere in agamids in the Hydrosaurus sailfin dragons). Their lifestyle requires their taking refuge in rock crevices or burrows when they’re not feeding, foraging, basking or interacting socially, a behavioural syndrome where a compressed body shape and defensive spiny tail are advantageous, and one that has evolved convergently in other iguanians – the American chuckwallas and ctenosaurs and Madagascan oplurines – and in the Australian Egernia skinks and in some African corylids (Pianka & Vitt 2003).
Caption: the tail of a deceased mastigure (probably U. acanthinurus), discovered in the Moroccan Sahara. Image: Darren Naish.
Herbivory in lizards works best at large size for the obvious reason of how much nutrition can be recovered (though it’s worth saying that there are many exceptions to this tendency: see Espinoza et al. 2004); it follows, then, that mastigures are relatively large compared to other agamids. I don’t know if there are any studies that do demonstrate this specifically, but the fact that most species are 25-45 cm long as adults does seem large, and the biggest species – the Egyptian or Leptien’s mastigure U. aegyptia – is positively enormous, reaching 75 cm on occasion and even more (specimens nearly 1 m long have been reported… can you imagine a mastigure this size? Amazing). It’s worth saying here that an especially large Paleogene lizard – Barbaturex from the middle Eocene of Myanmar, it perhaps reached 2 m in total – appears to be an especially close relative of Uromastyx (Head et al. 2013).
Caption: I was curious to know what a c 90 cm mastigure would look like compared to a person. The smaller of these silhouettes reveals the answer. Not as impressive as I was hoping. The larger lizard silhouette depicts the approximate size of the Eocene taxon Barbaturex, though we don't know that it had spiny whorls on its tail as shown in the illustration. The human figure is 1.7 m tall. Image: Darren Naish.
Mastigures are oviparous, females laying clutches of 6-20 elliptical eggs within a burrow. The hatchlings stay within the burrow for a few weeks, possibly even for months. The mother remains in attendance across this time and her burrow-guarding behaviour might be a form of parental care (directed both at the eggs and the hatchlings). Given that these lizards possess a symbiotic gut flora, the babies are presumably coprophagous. I’ve seen this stated informally but am not aware of a study that demonstrates it. Remember that tetrapods that possess a symbiotic gut flora must obtain it from their parents, and thus must eat their parent's dung early in life. Mm-mm.
Caption: an Egyptian spiny-tailed lizard U. aegyptia, as depicted in John Anderson's 1898 volume on the amphibians and reptiles of Egypt. Image: Anderson 1898.
Antiquity, taxonomy, biogeography. Having mentioned fossils, jaw fragments that appear to be from Uromastyx-like agamids (though not necessarily Uromastyx itself) are known from the Lower Eocene of Kyrgyzstan (Averianov & Danilov 1996) and hence establish an age of around 50 million years for this lineage. A number of Paleocene and Eocene lizards from Mongolia and China appear to be additional uromastycines. Rather younger, Oligocene fossils from the famous Jebel Qatrani Formation of the Fayum in Egypt’s Western Desert are sufficiently mastigure-like that they’ve been identified as ‘cf. Uromastyx’ (‘cf’ is an abbreviation of the Latin ‘confer’ and, when used in a taxonomic identification, basically means ‘we think that these fossils are so comparable to [insert taxon of interest] that they might belong to it, though we can’t be sure’). They date to the Lower Oligocene and hence are around 33 million years old (Holmes et al. 2010). There’s also a Lower Oligocene Uromastyx mastigure from France – yes, a European member of the group.
Caption: just one of the many uromastycine fossil jaw fragments from the Lower Eocene of Kyrgyzstan descibed by Averianov & Danilov (1996). These fossils - and others - demonstrate the antiquity of this group within Eurasia and show that it didn't arrive in the region after its Miocene collision with Africa. The scales bars = 1 mm. Image: Averianov & Danilov (1996).
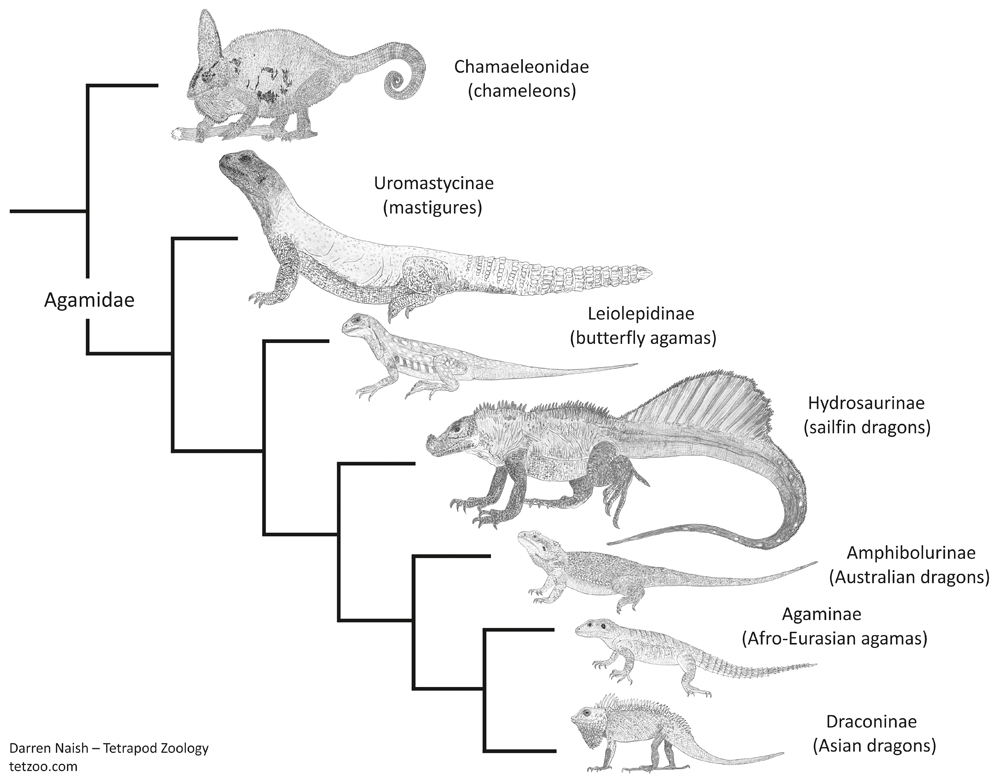
This antiquity is in keeping with the idea – made on the basis of their highly distinctive anatomy – that mastigures are ‘distinct enough’ from other agamids to be worthy of their own ‘subfamily’: Uromastycinae. This view derives support from those studies that have found or inferred mastigures to be a distinct lineage outside the clade containing all remaining crown-agamids (e.g., Frost & Etheridge 1989, Macey et al. 2000, Schulte et al. 2003, Pyron et al. 2013), and perhaps even outside the clade that includes chameleons and conventional agamids (Honda et al. 2000, Gauthier et al. 2012). That last result would push mastigure origins into the Cretaceous given amber fossils that seem to be stem-chameleons.
Caption: a phylogeny for agamids and their close kin, as recovered by Honda et al. (2000). Mastigures and butterfly agamas form a clade, and both are outside the clade that includes chameleons and 'Agamidae' of tradition. Like all of these sorts of diagrams, this was produced for my in-prep Vertebrate Fossil Record book, progress on which can be seen here. Image: Darren Naish.
Oh, you want Cretaceous stem-mastigures? In 2016, Apesteguía et al. (2016) described Jeddaherdan aleadonta from the Cenomanian of Morocco, and concluded that both this taxon and Gueragama sulamericana from the Upper Cretaceous of Brazil – both represented by partial lower jaws – are exactly that. Fossil evidence does, therefore, now back up the idea that these lizards were in existence before the end of the Cretaceous, and that acrodonts* (and thus iguanians more generally) had evolved at least some of their variation before the Cenozoic.
* Acrodonts (properly Acrodonta): the iguanian lizard clade that includes chameleons and agamids. They are named for their acrodont teeth: that is, those fused to the jawbones (though this condition is not fully developed across all members of the clade, and note that there are acrodont reptiles that are not part of Acrodonta).
Caption: the Cretaceous uromastycine Jeddaherdan aleadonta is known from the chunk of lower jaw shown here, depicted within a silhouetted skull of Uromastyx. The scale bar is in mm. Image: Apesteguía et al. (2016).
At least some studies find mastigures to form a clade with the east Asian butterfly agamas Leiolepis (e.g., Honda et al. 2000, Hugall & Lee 2004, Gauthier et al. 2012), both then being united within Leiolepidinae*. Butterfly agamas are fascinating for all sorts of reasons and I really should write about them at some point as well.
* There’s a long and complex argument over whether Leiolepidinae/Leiolepididae or Uromastycinae/Uromastycidae should win in a priority battle. Modern authors have tended to prefer the former, since it’s 1843 as opposed to 1863 for Theobald’s Uromastycidae. Anderson (1999) argued that the 1843 use of Fitzinger’s name cannot win this battle, since it was originally ‘Leiolepides’ and was not written in its ‘modern’ form by authors pre-1900.
Caption: mastigures and butterfly agamas have not been found to form a clade in all phylogenetic studies: in Pyron et al.'s (2013) study - this cladogram depicts the topology they recovered - the two are successively closer to remaining Agamidae. Note the taxonomic names they used for the agamid lineages. Like all of these sorts of diagrams, this was produced for my in-prep Vertebrate Fossil Record book, progress on which can be seen here. Image: Darren Naish.
Anyway: here I’ll say what I usually do and remind you that if these animals were mammals or birds they’d almost definitely be considered ‘distinct enough’ to warrant their own ‘family’, a decision that would require Agamidae of tradition to be split into several ‘families’ (I put these taxonomic ranks in quotes because they’re still effectively subjective). In addition to a mastigure family and butterfly agama family, there would be one for Hydrosaurus, one for the Australasian dragons (or amphibolurines), another for the Asian draconines, and so on. A few authors – most notably Scott Moody in his studies of the early 1980s – have at least separated mastigures and butterfly agamas from remaining agamids in a version of Theobald's ‘family’ Uromastycidae.
Caption: butterfly agamas (Leiolepis) do look mastigure-like in some features of the face (those tall ridges over the orbits especially), but are otherwise far slimmer, longer-limbed and without their other specialisations. The two groups may be closely related - though it still seems that they diverged during the Late Cretaceous, at least. Image: TheReptilarium, CC BY 2.0.
Macey et al. (2000) assumed an Indian origin for mastigures, in which case they’re among several tetrapod groups that followed an ‘Out of India’ dispersal route hypothesised elsewhere for ostriches and certain caecilians and frogs. But this is also contradicted by fossils, since Paleocene members of the lineage – if correctly identified and correctly dated – show that members of the lineage were living in Eurasia before India docked with Eurasia during the Eocene. The best model, therefore, might be one in which mastigures moved into Eurasia at the end of the Cretaceous.
Caption: an Eocene map depicting the planet as of around 40 million years ago. At this point, Afro-Arabia had not docked with Eurasia. But members of the mastigure lineage were already present in Eurasia and Afro-Arabia by the time. Image: the original version was used in Angst et al. (2013); this has been modified as per CC BY 2.5.
Tamar et al. (2018) posited an initial, middle Miocene diversification of the Uromastyx crown-group in south-east Asia followed by Afro-Arabian invasion and diversification. But note that this only applies to crown-group Uromastyx, not to the Saara + Uromastyx clade, nor to the mastigure lineage as a whole, and thus is not inconsistent with an earlier origin and diversification elsewhere.
Caption: Tamar et al. (2018) found Uromastyx to consist of two main clades, one mostly associated with the Arabian Peninsula and the borders of the Red Sea, one with the western Sahara. Saara forms the sister-group to Uromastyx. Image: Tamar et al. (2018).
Your regular dose of misanthropy. Finally, all is not well as goes the future of mastigures. As you might guess given my earlier mentions of the pet trade, the sad fact is that uncontrolled, indiscriminate and often illegal collection from the wild is a threat to many populations. Many people involved in the pet reptile trade – those at the sharp end where animals are taken from the wild and smuggled to other countries – have no scruples whatsoever as goes the ethical or managed treatment of animals, and if you don’t believe me look up articles on Anson Wong, the Malaysian wildlife smuggler known as the ‘Lizard King’ (a most inappropriate moniker, given that Kings are supposed to be worthy of respect or admiration).
Caption: I would love to see a large, spectacular mastigure in the wild. This mastigure (U. aegyptia microlepis), photographed in Al Anbar, Iraq, is a grand, magnificent animal. Image: U.S. Federal Government, Public Domain.
Mastigures have also been much used for food, medicine and as ritual objects (a cleaned mastigure body serves as a traditional baby’s bottle in Morocco, for example), all of which is fine (in theory) when harvesting is kept to sustainable levels… but less fine when exploitation begins to outstrip supply. Those mastigures that have been studied are declining or locally extinct across their range and all species are CITES listed as of 1977. Specifically, they’re on Appendix II of CITES, which refers to species that are not necessarily in immediate danger of extinction but do nonetheless require a control in their trade.
Caption: the large size and interesting appearance of many mastigure species - this is a captive U. aegyptia - has long made them appealing objects of trade and medicinal use, and as objects for the table too. Image: Darren Naish.
In some countries where these lizards occur it’s considered a rite of passage for young men to go out and kill as many mastigures as they can, and if you want verification for that you can find photos online where there are great piles of tens or even hundreds of dead mastigures in the backs of trucks. That’s depressing and vile behaviour. Like Anne Frank, I do think that people are essentially good but it’s difficult to maintain a rosy view of humanity when our stated aim seems to be the denuding of wild spaces of their animals.
On that depressing note, we move on.
Iguanian lizards have now been covered quite a few times at Tet Zoo. For previous articles see...
Harduns and toad-heads; a tale of arenicoly and over-looked convergence, December 2006
Ermentrude the liolaemine, February 2008
‘Cryptic intermediates’ and the evolution of chameleons, June 2008
Tell me something new about basilisks, puh-lease, January 2009
Amazing social life of the Green iguana, September 2012
The Squamozoic actually happened (kind of): giant herbivorous lizards in the Paleogene, June 2013
Leiosaurus: big heads, bold patterns, October 2013
Grassland earless dragons, January 2014
Australia, land of dragons (by which I mean: agamids) (part I), January 2014
Australia, land of dragons (part II), February 2014
What's With All These New Chameleon Names?, Part 1, February 2016
By the Horns of Trioceros, the Casque of Calumma, the Brood of Bradypodion--Chameleons, Part 2, February 2016
Palleon, Archaius, Kinyongia, Nadzikambia--The Last Chameleons, Part 3, March 2016
The Tropidurine Treerunners, December 2017
Refs - -
Apesteguía, S., Daza, J. D., Simões, T. R. & Rage, J. C. 2016 The first iguanian lizard from the Mesozoic of Africa. Royal Society Open Science 3: 160462.
Averianov, A. & Danilov, I. 1996. Agamid lizards (Reptilia, Sauria, Agamidae) from the Early Eocene of Kyrgyzstan. Neues Jahrbuch fur Geologie und Paläontologie, Monatshefte 1996 (12), 739-750.
Cooper, J. S. & Poole, F. G. 1973. The dentition and dental tissues of the agamid lizard Uromastyx. Journal of Zoology 169, 85-100.
Espinoza, R. E., Wiens, J. J. & Tracy, C. R. 2004. Recurrent evolution of herbivory in small, cold-climate lizards: breaking the ecophysiological rules of reptilian herbivory. Proceedings of the National Academy of Sciences 101, 16819-16824.
Frost, D. R. & Etheridge, R. 1989. A phylogenetic analysis and taxonomy of iguanian lizards (Reptilia: Squamata). University of Kansas, Museum of Natural History, Miscellaneous Publication 81, 1-65.
Gauthier, J. A., Kearney, M., Maisano, J. A., Rieppel, O. & Behlke, D. B. 2012. Assembling the squamate tree of life: perspectives from the phenotype and the fossil record. Bulletin of the Peabody Museum of Natural History 53, 3-308.
Holmes, R. B., Murray, A. M., Chatrath, P., Attia, Y. S. & Simons, E. L. 2010. Agamid lizards (Agamidae: Uromastycinae) from the Lower Oligocene of Egypt. Historical Biology 22, 215-223.
Honda, M., Ota, H., Kobayashi, M., Nabhitanhata, J., Yong, H.-S., Sengoku, S. & Hikida, T. 2000. Phylogenetic relationships of the family Agamidae (Reptilia: Iguania) inferred from mitochondrial DNA sequences. Zoological Science 17, 527-537.
Hugall, A. F. & Lee, M. S. Y. 2004. Molecular claims of Gondwanan age for Australian agamid lizards are untenable. Molecular Biology and Evolution 21, 2102-2110.
Macey, J. R., Schulte, J. A., Larson, A., Ananjeva, N. B., Wang, Y., Pethiyagoda, R., Rastegar-Pouyani, N. & Papenfuss, T. J. 2000. Evaluating trans-Tethys migration: an example using acrodont lizard phylogenetics. Systematic Biology 49, 233-256.
Robinson, P. L. 1976. How Sphenodon and Uromastyx grow their teeth and use them. In Bellairs, A. d’A. & Cox, C. B. (eds) Morphology and Biology of Reptiles. Academic Press (London), pp. 43-64.
Schulte, J.A., Valladares, J. P. & Larson, A. 2003. Phylogenetic relationships within Iguanidae inferred using molecular and morphological data and a phylogenetic taxonomy of iguanian lizards. Herpetology 59, 399-419.
Tamar, K., Metallinou, M., Wilms, T., Schmitz, A., Crochet, P.-A., Geniez, P. & Carranza, S. 2018. Evolutionary history of spiny-tailed lizards (Agamidae: Uromastyx) from the Saharo-Arabian region. Zoologica Scripta 47, 159-173.
Throckmorton, G. S. 1976. Oral food processing in two herbivorous lizards, Iguana iguana (Iguanidae) and Uromastix [sic] aegyptius [sic] (Agamidae). Journal of Morphology 148, 363-390.
Wilms, T. Böhme, W., Wagner, P., Lutzmann, N. & Schmitz, A. 2009. On the phylogeny and taxonomy of the genus Uromastyx Merrem, 1820 (Reptilia: Squamata: Agamidae: Uromastycinae) – resurrection of the genus Saara Gray, 1845. Bonner Zoologische Beiträge 56, 55-99.