The fossil record is a cruel and fickle mistress, and there are a vast many fossil animals for which key data on lifestyle and biology is simply not preserved, or – at least – not known. Yet…
Azhdarchid Progress, a Personal View
The Quetz Monograph Lives and Other News on Azhdarchid Pterosaurs
Baby Pterosaurs Were Excellent Fliers and Occupied Different Niches From Their Parents
New Perspectives on Pterosaur Palaeobiology, the TetZoo Review
Did Dinosaurs and Pterosaurs 'Glow'? Extinct Archosaurs and the Capacity for Photoluminescent Visual Displays
One of many exciting discoveries made in tetrapod biology in recent decades is that UV-sensitive vision is not just a thing that exists, but a thing that’s widespread.
Caption: would a live dinosaur - like this heterodontosaur - look utterly different if its tissues were photoluminescent? Brian Engh explored this possibility in this excellent piece of art, included in Woodruff et al. (2020). Image: Brian Engh.
We’ve known since the early 1980s that at least some birds can detect UV wavelengths, and research published more recently has demonstrated its presence in lizards of disparate lineages, in turtles, rodents and, most recently, amphibians. Some of these animals use their UV-sensitive vision to find food (like pollen-rich flowers) and perhaps even to navigate their environments (UV-sensitive vision in certain forest-dwelling birds might enhance their ability to see certain kinds of leaves, for example).
That’s great, but what’s even more surprising – though maybe it shouldn’t be – is that markings and tissue types in some of these animals are visible to other animals with UV-sensitive vision. Furthermore, some tissue types are able to absorb UV and re-emit it within part of the spectrum visible to we humans. It’s this aspect of the UV story – the possibility that UV is absorbed and emitted as visible light (typically blue light) – that we’re talking about hereon, not UV-sensitive vision. Note that the terms used for this phenomenon are slightly contentious among relevant experts. Most agree that the right term is fluorescence whereas others (including my colleague Jamie Dunning) argue that we should use the more specific photoluminescence. I have no proverbial dog in this fight but am going to stick with photoluminescence here seeing as it’s the one we used in the relevant paper.
Caption: in 2018, Jamie Dunning and colleagues reported the discovery of photoluminescence in puffins. Image: (c) Jamie Dunning.
The discovery of photoluminescence in animals is evidently of broad general interest, and I can make this assertion because several recent studies reporting its occurrence have received an unusual amount of public interest. Dunning et al.’s (2018) report on its occurrence in the brightly coloured bill plates of puffins, for example, proved a really popular discovery (Wikinson et al. (2019) followed up with a subsequent study on the keratinous horns of rhinoceros auklets), as did Prötzel et al.’s (2018) discovery of photoluminescent bones in chameleons. Remarkably, Prötzel et al. (2018) were able to show that the ‘glowing’ bones of these lizards are visible through the skin. At the time of writing, a study reporting widespread photoluminescence in living amphibians has just appeared, and it too has received a fair amount of general interest.
Here it’s worth making a critical point on the popularity of these studies in the popular media. There’s no doubt that this stuff is interesting, and certainly of relevance to biologists at large (for one thing, knowing about the distribution of fluorescence/photoluminescence could have all kinds of implications for surveying and collecting). But there’s concern that the studies are being framed in the wrong way, and that more thorough vetting is needed, in places. Also worth noting is that what role photoluminescence actually has to the animals that emit it is controversial, since some workers argue (a) that its visual signalling role hasn’t been sufficiently tested for, and (b) it may simply be too subtle to be of much use to the animals in which it’s present. Keep this in mind when reading the following!
Caption: Prötzel et al.’s (2018) bone-glow research on chameleons shows that the photoluminescing bones of these lizards were actually visible through the skin. Image: David Prötzel.
These caveats notwithstanding, if UV-themed visual displays are widespread in tetrapods, those of us interested in fossil animals are presented with an interesting set of possibilities. We already think that the many extravagant structures of non-bird dinosaurs and pterosaurs – they include cranial horns, crests and casques as well as spikes, spines, sails, bony plates and so on – functioned predominantly in visual display. Could they also have been photoluminescent, and could this have then been used to enhance the display function of the structures in question?
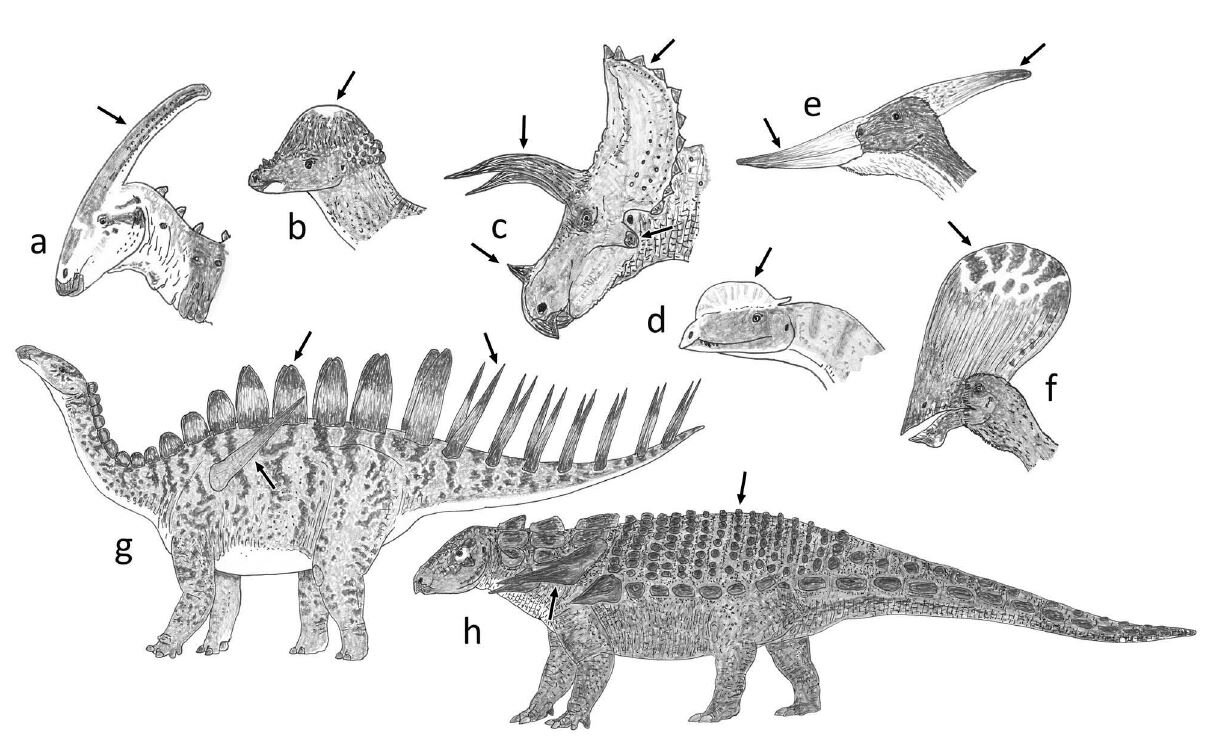
Caption: dinosaurs and pterosaurs are of course notable for their remarkable variety of what I term extravagant structures, a selection of which are depicted here. (a) Parasaurolophus, a hadrosaurid ornithopod. (b) Pachycephalosaurus. (c) Triceratops, a ceratopsid ceratopsian. (d) Dilophosaurus, a theropod. (e) Pteranodon and (f) Tupandactylus the pterodactyloid pterosaurs. (g) Miragaia the stegosaur. (h) Edmontonia the nodosaurid ankylosaur. From Woodruff et al. (2020), images by Darren Naish.
In a brand-new paper published this week in Historical Biology (or on its website, anyway), Cary Woodruff, Jamie Dunning and I set out to consider this very question (Woodruff et al. 2020). At the risk of spoiling the surprise I’ll say that we don’t provide a hard or definitive answer; our aim instead is to bring attention to the possibility that photoluminescence might have been present in some of these animals. We encourage the testing of this possibility and suggest some specific ways in which this testing might be performed. Of incidental interest is that our collaboration evolved from a Twitter discussion (which is currently findable here).
Caption: a palaeontologist ponders new papers on photoluminescence, and then gets talking to one of the relevant researchers. And I chimed in as well, sorry. The rest is history…
I should also add that our idea isn’t especially new. Ever since UV-sensitive vision was first reported in birds back in the 1980s, the idea that extinct dinosaurs might have made use of photoluminescence has been mooted (though, let me make the point again: you don’t need UV-sensitive vision to see photoluminescence). I’ve incorporated photoluminescence into more than one dinosaur-themed media project, most recently Dinosaurs in the Wild.
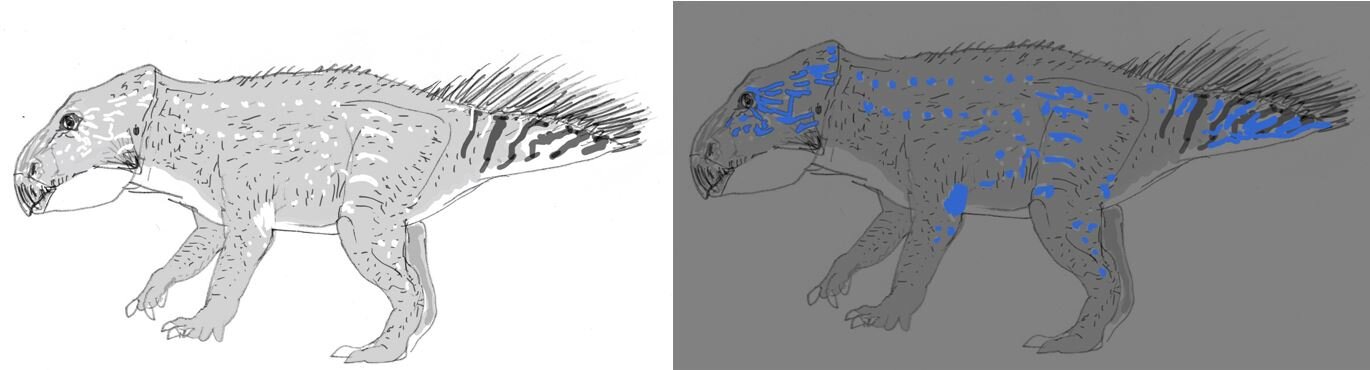
Caption: the idea that Mesozoic dinosaurs might have been exploiting photoluminescence isn’t altogether new. Here are rough sketches I produced depicting the concept of a photoluminescent Leptoceratops produced for the travelling visitor experience Dinosaurs in the Wild. Image: Darren Naish.
A few specific points are worthy of attention. Above, I mentioned Prötzel et al.’s (2018) chameleon-themed ‘bone glow’ study. Bone-based photoluminescence has also been reported in frogs, specifically in the Brachycephalus pumpkin toadlets (Gouette et al. 2019). Could those dinosaurs superficially similar to chameleons (namely ceratopsians: like some chameleons, they have bony frills and prominent horns) also possess bone-based photoluminescence and, if so, could they exploit it in chameleon-like fashion? Well, probably not, mostly because the much larger size of these dinosaurs means that their skin was too thick for this to work (Woodruff et al. 2020).
Caption: for fun, let’s use toy ceratopsians rather than the real things. Could these dinosaurs have had ‘glowing’ bones as modern chameleons do? No, almost certainly not. Image: Darren Naish.
One of the most unusual things about non-bird dinosaurs possessing extravagant structures is that males and females are extremely similar (albeit not necessarily identical) with respect to the form and proportional size of said structures. As regular TetZoo readers might recall from several articles published here within recent years (see links below), some workers interpret the extravagant structures of Mesozoic dinosaurs as functioning within a model of species recognition. According to this model, the structures function as banners used to signal membership of whatever the respective species is. I don’t think that this is valid for a bunch of reasons and in fact I don’t think that extravagant structures have an important role in species recognition at all (Hone & Naish 2013, Knell et al. 2013). An alternative model posits that extravagant structures mostly have an intraspecific function, work as sociosexual signals of reproductive quality, and evolved within the context of sexual selection. This is the model that I and my colleagues support (Hone et al. 2011, Knell et al. 2012, 2013, Hone & Naish 2013), and a lengthy debate that’s been thrashed out in the literature over the past decade pits species recognition and sexual selection as opposing schools of thought.
Caption: at left, mutual sexual selection at play in the Great crested grebe, as illustrated by Julian Huxley in 1914. At right, cover of the famous issue of TREE which includes Knell et al.’s (2012) seminal review.
But if this is so, why is it that ostensible males and females in the dinosaur species concerned are monomorphic: that is, they have similar extravagant structures? Back in 2011, Dave Hone, Innes Cuthill and I argued that these animals might have evolved their extravagant structures within the context of mutual sexual selection (Hone et al. 2011), this being the strategy where both males and females use their extravagant structures in sociosexual display. But while we know that extant monomorphic animals really are monomorphic, we’re not sure that this is (or was) the case for extinct ones: it could still be that their structures differed in hue, colour or some other visual property. If we’re speculating about the possible presence of photoluminescence in extinct archosaurs, the possibility exists that “monomorphic elaborate structures in pterosaurs and non-bird dinosaurs were not monomorphic in life” but differed in how they photoluminesced (Woodruff et al. 2020, p. 5). We were inspired by the sexually dimorphic photoluminescence of chameleons and Brachycephalus frogs.
Caption: could the in-situ, fully intact armour of ankylosaurs like that of the amazing holotype of Borealopelta, shown here, give insight into the potential of photoluminescence in these animals? Image: CC SA 4.0, original here.
Finally… speculating about the presence of photoluminescence is all very well and good, but can we test for it? In those cases where part of the integument is preserved, we can, by shining blacklights at the respective specimens. The problem, however, is that we might not be seeing the original light-emitting properties of the animal. Seemingly positive results might be a consequence of the fact that various tissues (bone included), minerals and preservatives fluoresce under UV (Woodruff et al. 2020).
As a preliminary test, we looked at the osteoderms of the spectacularly preserved ankylosaurs Borealopelta and Zuul under UV light… we did get results, but it’s difficult to know what, if anything, these results tell us about any condition present in life (Woodruff et al. 2020). I should add that people have been shining blacklights at fossils for a long time and seeing all kinds of interesting results (hat-tip to the pioneering work of Helmut Tischlinger); in no way are we implying that we’re anything like the first to do this.
Caption: people have been examining fossils with UV light for decades. These images show the Jurassic pterosaur Bellubrunnus roethgaengeri, illuminated via the use of UV. Image: Hone et al. 2012 (original here).
And that about wraps things up for now. As will be clear, our paper is not much more than a preliminary set of speculations and suggestions for further work, and isn’t intended to be an in-depth analysis of the proposal. But – as I see it – that’s ok: the scientific literature really shouldn’t be considered focused on results alone, since review, discussion and valid speculation are valuable and worthy too. I hope you agree.
UPDATE (adding 4th March 2020): this article has been somewhat modified relative to its original version, since a misunderstanding on my part meant that I was previously describing photoluminescence as a phenomenon especially relevant to animals with UV-sensitive vision. Substantial thanks to Michael Bok for his interest and assistance and for sending comments which enabled me to modify the article.
For previous TetZoo articles on the biology and life appearance of Mesozoic dinosaurs and pterosaurs, see (as usual now, linking to wayback machine versions due to vandalism and paywalling of ver 2 and 3)…
Zuniceratops and the early acquisition and alleged dimorphism of ceratopsian brow horns, April 2009
Necks for sex? No thank you, we’re sauropod dinosaurs, May 2011
Did dinosaurs and pterosaurs practise mutual sexual selection?, January 2012
Sexual selection in the fossil record, September 2012
Dinosaurs and their ‘exaggerated structures’: species recognition aids, or sexual display devices?, April 2013
Refs - -
Dunning, J., Diamond, A. W., Christmas, S. E., Cole, E. L., Holberton, R. L., Jackson, H. J., Kelly, K. G., Brown, D., Rojas Rivera, I. & Hanley, D. 2018. Photoluminescence in the bill of the Atlantic Puffin Fratercula arctica. Bird Study 65 (4), 1-4.
Goutte, S., Mason, M.J., Antoniazzi, M.M., Jared, C., Merle, D., Cazes, L., Toledo, L.F., el-Hafci, H., Pallu, S., Portier, H., Schramm, S., Gueriau, P. & Thoury, M. 2019. Intense bone fluorescence reveals hidden patterns in pumpkin toadlets. Scientific Reports 9, 5388.
Prötzel, D., Heß, M., Scherz, M. D., Schwager, M., van’t Padje, A. & Glaw, F. 2018. Widespread bone-based fluorescence in chameleons. Scientific Reports 8, 698.
Wilkinson BP, Johns ME, Warzybok P. 2019. Fluorescent ornamentation in the Rhinoceros auklet Cerorhinca monocerata. Ibis 161, 694-698.
Postcranial Palaeoneurology and the Lifestyles of Pterosaurs
Regular readers will know that I – with colleagues – publish fairly regularly on azhdarchoid pterosaurs, the very special pterosaur group that includes the short-faced tapejarids, the sometimes gigantic, long-jawed azhdarchids and a few groups seemingly intermediate between these two. Azhdarchoids are quite obviously the best and most interesting of the pterosaurs. And back in 2013, I and colleagues published a description and analysis of a new species from the Early Cretaceous of the Isle of Wight known only from a three-dimensional pelvis and some associated vertebrae. We called it Vectidraco daisymorrisae, its name honouring Daisy Morris, the young woman who discovered it (Naish et al. 2013).
The Vectidraco daisymorrisae holotype (NHMUK PV R36621) in (A) left lateral, (B) right lateral, (C) dorsal and (D) ventral views, and - at right - shown in anatomical position as per the animal's presumed profile in life. Image: figures from Naish et al. (2013).
Enough is known of Vectidraco for us to make some determination as goes what sort of pterosaur it is (it seems to be a tapejarid or tapejarid-like azhdarchoid), and we can also say interesting things as goes its size and degree of skeletal pneumatisation (Naish et al. 2013). It’s well preserved enough that quite a few other things can be done with it as well. Last year Rachel Frigot used it as a model in the determination of pelvic and hindlimb musculature (Frigot 2017). And, as part of her PhD work on pterosaur pneumaticity and anatomy, my colleague Liz Martin-Silverstone sought to do some neat science with it as well. This work has just been published (Martin-Silverstone et al. 2018), and that’s why we’re here today.
My friend and colleague Dr Liz Martin-Silverstone, at work in the field (at left, Liz is finding fossils in a river in Romania) and in a museum exhibition at right (Liz is standing next to an exhibition panel all about her work. Let's not talk about that weird silhouette at upper right...). Images: Darren Naish.
Much of Liz’s work has involved CT-scanning (you can read about her own adventures here on her blog) and the relationship between pneumatisation, mass and flight. Vectidraco is at the other end of the scale from many of the pterosaurs that Liz has worked on (it was a small pterosaur with a wingspan likely less than 1 m as an adult) and was readily available, so it seemed sensible to incorporate it into her work. We scanned the specimen at its home (the Natural History Museum, London), and compared the results with those obtained from other pterosaurs we had to hand: namely, the ornithocheirids* Anhanguera and Coloborhynchus, scanned variously at Stony Brook University Hospital (thanks to Pat O’Connor for that data) and at the µ-VIS (pronounced ‘mu-vis’) X-Ray Imaging Centre at the University of Southampton (Martin-Silverstone et al. 2018). And we got pretty good results.
* Ornithocheirids: the mostly marine, long-jawed, long-winged pterodactyloid pterosaur group named for Ornithocheirus from the 'middle' Cretaceous of the UK. The group names Anhangueria and Anhangueridae refer to the same group... views differ on which taxonomic system we should adopt.
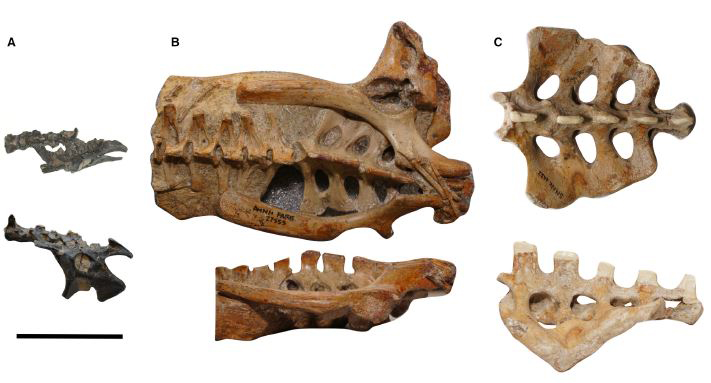
Pelvic regions of the three pterosaurs included in our study, to scale: (A) Vectidraco daisymorrisae holotype NHMUK PV R36621, (B) Anhanguera specimen AMNH FARB 22555, (C) Coloborhynchus robustus specimen SMNK PAL 1133. Scale bar = 50 mm. Image: Martin-Silverstone et al. (2018).
The first interesting thing to note is that the work corrects, updates and augments various anatomical details I reported in the initial description of Vectidraco (Naish et al. 2013). Bony openings that I interpreted as pneumatic foramina turn out to be foramina for spinal nerves (properly termed intervertebral foramina), and convex transverse ridges present on the sides and undersides of some of the vertebrae are misidentified intervertebral junctions. Cool – it’s good to learn more. The identification of intervertebral foramina is not a big deal at all given that these structures are ubiquitous in tetrapods but it's worth bringing attention to them given that they’re virtually unmentioned elsewhere in the pterosaur literature.
The Vectidraco holotype is one of those wonderful specimens that preserves a great many neat little anatomical details - look at these various pneumatic cavities on the T-shaped post-acetabular process on the posterior part of the ilium. Waitaminute.... aren't all the specimens like this? Image: Darren Naish.
And if you’re wondering why I and my colleagues didn’t CT-scan the specimen the first time around and get this stuff correct on our first, 2013 attempt, it’s because CT-scanning requires money and virtually everything I do has been, and is, unfunded.
Anyway… what else could we do with the CT-scan data? Well…
During the late 1980s and 90s, Emily Giffin (later Emily Buchholtz) published several papers in which she used data from the size of the neural canal in the vertebrae of fossil tetrapods to make inferences about nerve size, the size then serving as a proxy for degree of innervation, this then serving as a guide to things like limb function and posture (Giffin 1989, 1990, 1992, 1995a, b). Her studies looked variously at non-bird theropods, extinct crocodylians and fossil pinnipeds, and she reported encouraging results. Non-bird theropods with large hands, to take one example, possessed neural canals in the corresponding part of the spine that were proportionally large, and hence suggestive of the well-developed nervous anatomy we would expect for animals that regularly used their hands in grabbing, piercing and tearing (Giffin 1995a). Her work has inspired other researchers to use the same (or similar) techniques on plesiosaurs and fossil raptors (again, here’s your helpful reminder that I will only ever use this term in the correct fashion. It applies to hawks, eagles and falcons and has done since the 1800s at least).
In a series of really interesting papers, Emily Giffin linked neural canal size with form and function in diverse tetrapods. This graph (from Giffin 1995b) shows how birds flying and flightless differ as goes the position of the largest parts of their spinal cords. The ostrich (Struthio) lacks a large spinal cord section in the anterior (brachial) part of its spinal column. Image: Giffin (1995b).
Several caveats make this technique far from fool-proof (Giffin 1995a). With these things in mind, we wondered if data from pterosaurs might be informative as goes ideas on their ecology and lifestyle. What we found is that Vectidraco has an unusually large neural canal in its sacral region compared to Anhanguera, indicating that it therefore had a proportionally large spinal cord (and lumbosacral plexus) in its lumbosacral region. Anhanguera and Coloborhynchus both had enlarged neural canals in the area corresponding to the brachial plexus, larger than the neural canals in their sacral regions. Vectidraco’s shoulder region is entirely unknown at the moment so we couldn't make any comparison here.
Neural canal cross-sectional area in our three pterosaur taxa: when normalised for centrum size, Vectidraco has a proportionally large neural canal. This composite image incorporates figures from Martin-Silverstone et al. (2018) but was produced by the Palaeontological Association. Image: Martin-Silverstone et al. (2018).
Taken together this suggests the following: evidence for a large brachial enlargement in ornithocheirids is consistent with the idea (based on their long, high-aspect wings and small hindlimbs) that they were highly aerial animals, while the large sacral neural canal in Vectidraco indicates that it was more proficient at terrestrial locomotion than Anhanguera. There are already indications from pelvic morphology, hindlimb size and so on that azhdarchoids and ornithocheirids were doing very different things in terms of ecology and behaviour (Witton & Naish 2008, 2015, Witton 2013, Naish & Witton 2017), so this matches what we might predict.
Vectidraco could almost certainly fly well, as shown at left. But - like many, most or all azhdarchoids - it was likely a proficient and regular terrestrial walker as well, as shown at right. Image: Mark Witton (left), Darren Naish (right).
So far so good. But the complication comes from the second ornithocheirid we looked at: Coloborhynchus. Oh, here I’ll avoid the whole mess concerning the taxonomy of Anhanguera and Coloborhynchus. All I’ll do for now is say that “it’s complicated” and promise that I’ll come back to it in the near future. Anyway… the Coloborhynchus specimen we analysed is not like our Anhanguera specimen as goes the proportional size of the neural canal in its lumbosacral region -- it lacks a distinct lumbosacral enlargement but is superficially Vectidraco-like in having a larger neural canal in the relevant region (Martin-Silverstone et al. 2018). This is an unexpected result. Does it mean that some ornithocheirids were far more terrestrially capable than others, this perhaps reflecting niche differentiation or some other form of variation? Or does it mean that some ornithocheirids were far more aerially specialised than others and that the default condition was to have a larger sacral neural canal? Or does it mean that there’s something else we haven’t accounted for? (example: maybe some of these pterosaurs had enlarged neural canals due to pneumatisation in the neural canal? Yes, air sacs dorsal to the spinal cord. This is a thing). I don’t think it means that Coloborhynchus-type pterosaurs were as terrestrially proficient as Vectidraco, nor that our ideas on said terrestrial proficiency in azhdarchoids like Vectidraco are bogus, given the fact that Vectidraco appears to have a lumbosacral enlargement while the ornithocheirids do not, and that azhdarchoids like Vectidraco possess weird features indicative of terrestrial specialisation (like the giant, T-shaped postacetabular process on the ilium) lacking in ornithocheirids. But it’s clear that more data and more work is needed, as usual with these sorts of things.
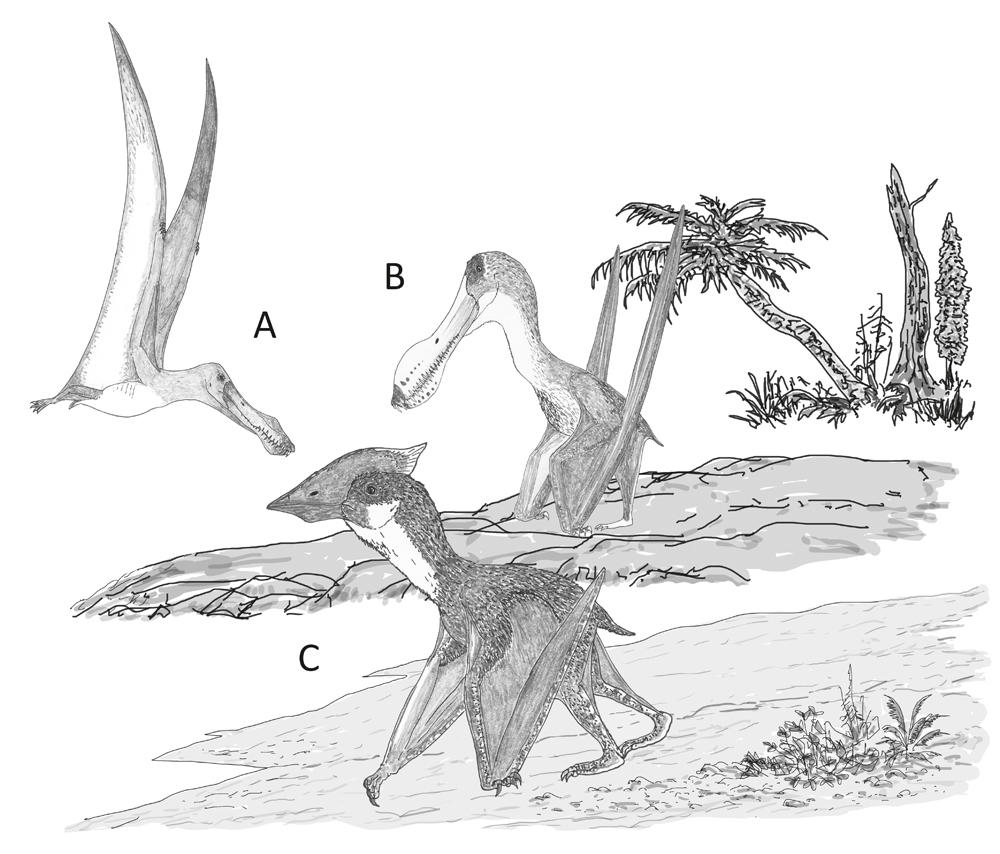
Behaviour speculatively inferred for the pterosaurs incorporated in our study. (A) A dedicated aerial lifestyle involving little terrestrial behaviour, as per Anhanguera; (B) reasonable terrestrial abilities in an animal otherwise very similar to its close, highly aerial relatives, as per Coloborhynchus; (C) proficient and regular terrestrial behaviour in an animal that routinely feeds and forages on the ground, as per Vectidraco. Image: Darren Naish.
It’s also important to remember that this data – this work as a whole – is complimentary to other studies. CT scan data on neural canal size provides nothing like a ‘Rosetta Stone’ on behaviour or lifestyle, and the more we learn about anatomy and form-function correlation, the less likely it seems that such things exist. We already have lots of reasons for thinking that azhdarchoids were better adapted for terrestriality and life in cluttered, inland settings than were many other pterosaurs, and likewise there are excellent reasons for thinking that ornithocheirids were aerial specialists and something like frigatebirds as goes ecology and lifestyle (though seemingly capable of swimming and perhaps diving, in contrast to the more piratical frigatebirds). So, this work is part of the dataset, part of the puzzle. And this is effectively an opening foray in this most intriguing area.
That’s where we’ll end for now. More on pterosaurs here soon!
For previous Tet Zoo articles on pterosaurs, see...
Shemhazai and other flightless pterosaurs, September 2008
Come back Lank, (nearly) all is forgiven, September 2008
Giant pterosaurs invade London, Summer 2010, July 2010
Pterosaurs, err, indoors (the Summer 2010 exhibition), July 2010
The Cretaceous birds and pterosaurs of Cornet: part II, the pterosaurs, September 2010
What does it feel like to get bitten by a ground hornbill, I hear you ask?, June 2011
A new azhdarchid pterosaur: the view from Europe becomes ever more interesting, Jan 2013
Daisy’s Isle of Wight Dragon and why China has what Europe does not, March 2013
In Rio for the 2013 International Symposium on Pterosaurs, May 2013
Quetzalcoatlus: the evil, pin-headed, toothy nightmare monster that wants to eat your soul, August 2013
Were azhdarchid pterosaurs really terrestrial stalkers? The evidence says yes, yes they (probably) were, December 2013
Some Azhdarchid Pterosaurs Were Robust-Necked Top-Tier Predators, February 2017
Refs - -
Frigot, R. 2017. Pelvic musculature of Vectidraco daisymorrisae and consequences for pterosaur locomotion. In Hone, D. W. E., Witton, M. P. & Martill, D. M. (eds) New Perspectives on Pterosaur Palaeobiology. Geological Society, London, Special Publications Special Publications 455, 45-55.
Giffin, E. B. 1989. Gross spinal anatomy and limb function in living and fossil reptiles and birds. American Zoologist 29, 181A.
Giffin, E. B. 1990. Gross spinal anatomy and limb use in living and fossil reptiles. Paleobiology 16, 448-458.
Giffin, E. B. 1992. Functional implications of neural canal anatomy in recent and fossil marine carnivores. Journal of Morphology 214, 357-374.
Giffin, E. B. 1995a. Functional interpretation of spinal anatomy in living and fossil amniotes. In Thomason, J. J. (ed) Functional Morphology in Vertebrate Paleontology. Cambridge University Press, pp. 235-248.
Giffin, E. B. 1995b. Postcranial paleoneurology of the Diapsida. Journal of Zoology 235, 389-410.
Martin-Silverstone, E., Sykes, D. & Naish, D. 2018. Does postcranial palaeoneurology provide insight into pterosaur behaviour and lifestyle? New data from the azhdarchoid Vectidraco and the ornithocheirids Coloborhynchus and Anhanguera. Palaeontology 2018, 1-14. doi: 10.1111/pala/12390
Naish, D., Simpson, M. I. & Dyke, G. J. 2013. A new small-bodied azhdarchoid pterosaur from the Lower Cretaceous of England and its implications for pterosaur anatomy, diversity and phylogeny. PLoS ONE 8 (3): e58451.
Witton, M. P. 2013. Pterosaurs. Princeton University Press, Princeton & London.