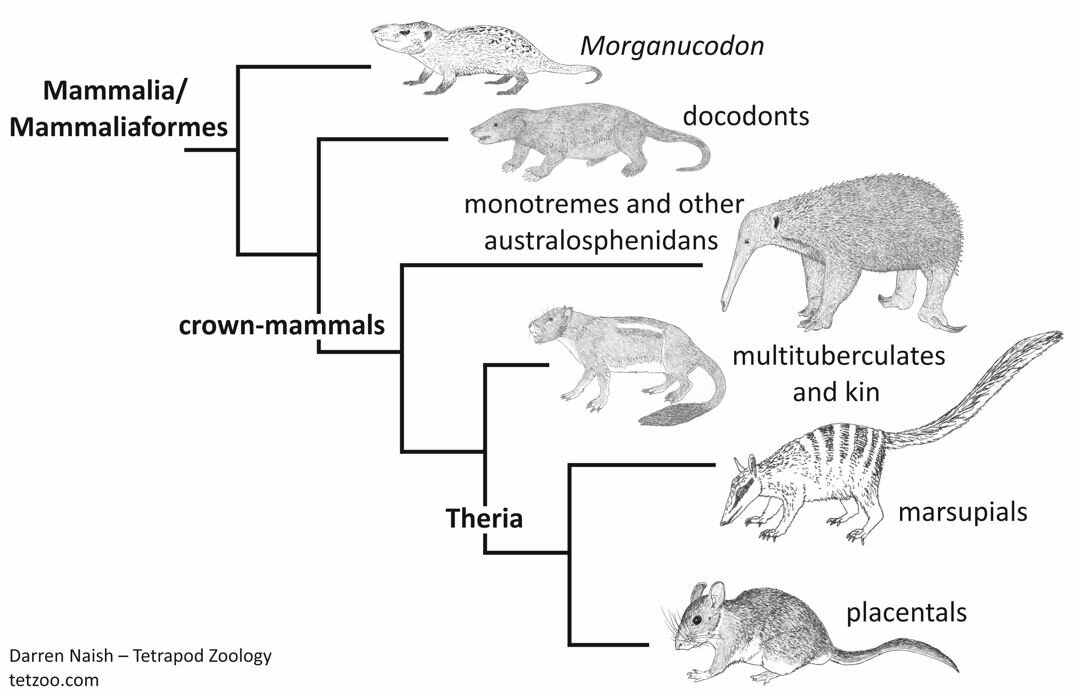
If you know anything about mammals, you’ll know that crown-mammals – modern mammals – fall into three main groups: the viviparous marsupials and viviparous placentals (united together as therians), and the egg-laying monotremes. The fact that monotremes lay eggs is familiar to us today, but of course it was a huge surprise when first discovered. There’s a whole story there which I won’t be recounting here.
Caption: were non-therian mammals like morganucodontids, docodonts and multituberculates laying eggs like monotremes (represented here by the echidna Zaglossus) or giving birth to pink little babies like placental mammals do? Image: Darren Naish (images from the in-prep textbook project).
So far so good, but which reproductive strategy was used by those mammals and mammal ancestors – the whole lot are termed stem-mammals – outside the crown, and by those mammals that are within the crown, but closer to therians than are monotremes? Were they laying eggs like monotremes, or are monotremes unusual relative to these animals, or what? Here’s the issue I want to discuss, and I want your thoughts.
Caption: a highly simplified cladogram which depicts the relationships relevant to this article. Image: Darren Naish.
The fact that egg-laying is ancestral for amniotes and present in monotremes suggests that oviparity should be the ‘assumed’ condition for all relevant animals outside Theria. Ergo, non-mammalian synapsids like Dimetrodon, archaic therapsids like dinocephalians and therocephalians, near-mammals like tritheledontids and tritylodontids, animals relatively close to crown-mammals like, say, morganucodonts and docodonts, and even mammals which are within the crown but outside of Theria – like the haramiyadans and multituberculates of the Mesozoic and Paleogene – should all have laid eggs. Right?
Caption: everyone’s favourite volaticothere reconstruction; it’s by Chuang Zhao and Lida Xing. Should we imagine this animal as an egg-layer? I’ve said yes, but I could be very wrong. Image: (c) Chuang Zhao and Lida Xing.
Surprisingly little has been written about this question (at least, so far as I can see). A few years ago I recall a reader of the Ask A Biologist website asking if little Volaticotherium of the Jurassic – famous for its gliding membranes – would have laid eggs. Based on the assumption that oviparity was the normal condition for mammals in this section of the tree, I said yes (the relevant page is here). A few authors have implied support for this position (Sharman 1970, Hopson 1973). I looked around for other opinions and found essentially nothing.
Caption: there are a few - not that many - books on stem-mammal evolution out there, all of which have their strengths and weaknesses. Image: Darren Naish.
Indeed, the starting and ending point for discussion of this issue seems to be Zofia Kielan-Jaworowska’s paper of 1979 (Kielan-Jaworowska 1979), and a recounting of her arguments in the 2004 book Mammals From the Age of Dinosaurs: Origins, Evolution and Structure (Kielan-Jaworowska et al. 2004). Of those other books that discuss the deep history of mammalian character evolution (e.g., Kemp 1982, Kermack & Kermack 1984, Kielan-Jaworowska 2013), most scarcely even mention the terms ‘eggs’, ‘oviparity’ and ‘viviparity’. The authors of these works were simply interested in other things. Well, and constrained by a lack of relevant data.
Caption: most people interested in the history of palaeoart and the popularisation of dinosaur-themed palaeontology are familiar with John McLoughlin’s Archosauria. Less well know is its successor, Synapsida.
An exception is John C. McLoughlin’s Synapsida: A New Look Into the Origins of Mammals (McLoughlin 1980), the sister volume to his better known Archosauria of 1979 (McLoughlin 1979). McLoughlin was (and is) a writer, not a scientist, and I haven’t seen specialists on synapsid evolution say positive things about his book. Anyway, therein he makes the suggestion that the transition from oviparity to viviparity might have occurred among ‘pantotheres’, a group of Mesozoic mammals (no longer regarded as a clade) essentially thought to include docodonts and dryolestoids* and thus be within crown-mammals and close to the ancestry of Theria. If McLoughlin is right in speculating the origin of viviparity at this point, it could mean that oviparity was present across all extinct australosphenidans, in the so-called eutricodontans (the gobiconodontids, triconodontids and so on), and in multituberculates and other allotherians. This corresponds with the expectation I outlined at the start of the article.
* The historical use of the term ‘pantothere’ is way more complicated than that, but we need not worry about that here.
Viviparity is not the ‘end member’ of a sequence. Another published discussion was provided by Guillette & Hotton (1986). They proposed the existence of substantially more variability and flexibility in reproductive styles across non-therians than did McLoughlin: they suggested that viviparity could have evolved as early as the Late Permian, but that oviparity might have been retained across several lineages. They didn’t make any specific predictions about any given groups, but implied that the existence of oviparity in monotremes might not necessarily mean that the lineages surrounding them in phylogeny were oviparous too (Guillette & Hotton 1986).
Caption: there aren’t many books which cover the biology of stem-mammals, and Hotton et al. (1986) is invaluable if you’re interested in this subject. Guillette & Hotton’s (1986) paper on stem-mammal reproduction is within.
Two things are worth saying here, both of which are vague and arm-wavy. (1) It somehow feels right to me that we may indeed see a variation in reproductive strategies across the animals involved in this transition. They were a diverse bunch, living in diverse ways in diverse ecological settings, and it’s wrong to think that viviparity is a ‘superior’ or ‘inevitable’ behaviour that’s the ‘end member’ of a sequence that starts with oviparity. The viviparity seen in therians might be present in these animals because it’s a reproductive strategy that one synapsid clade became locked into at the start of their history, not because it’s obviously superior, and maybe viviparity evolved elsewhere, here and there, across Synapsida. This would be reminiscent of the pattern seen in squamates, chondrichthyans, actinopterygians, coelacanths, amphibians and even arthropods. (2) It may be wrong to think – as we mostly do – that the oviparity present in monotremes is (a) primitive and (b) representative of an ancestral condition for mammals. As is well known and oft-mentioned, monotremes are highly specialised. Could their oviparity be an unusual specialisation, not just an inherited, ancestral feature? Guillette & Hotton (1986) made a nod to this, stating that “[monotremes do not] necessarily indicate that the cynodont lineage was exempt from this propensity to experiment [with viviparity and oviparity], for they may be merely a surviving trace of cynodont diversity” (p. 246).
Caption: viviparity has evolved on a great many occasions within squamates, yet we don’t think of the viviparous species as the ‘end’ members of an evolutionary sequence. Maybe we should think about synapsid viviparity in the same way. Here are but a few viviparous squamates. Clockwise from upper left: Common slow-worm Anguis fragilis, Common European adder Vipera berus, Cunningham’s skink Egernia cunninghami. Images: Neil Phillips (used with permission); Benny Trapp, CC BY 3.0 (original here); Donald Hobern, CC BY 2.0 (original here).
If variation in reproductive styles was the case across non-therian mammals, it could be that lineages were doing whatever it is they were doing according to their selective demands. This makes sense given what we see in living animal groups. There are several cases in squamates (carphodactyline geckos, vipers, Asian pipe snakes, some anguids) where it’s been proposed that viviparity isn’t a ‘dead end’ for the evolution of variation, and where oviparity might have evolved from viviparous ancestors (Lee & Shine 1998)*, plus we know of lineages and even species where both oviparity and viviparity are present (Lee & Shine 1998, Surget-Groba et al. 2001). If this sort of variation was the case in non-therian synapsids, it makes it harder to predict what might have been happening in any one extinct lineage or species. And the oviparity of monotremes might not tell us anything about the strategies used by other australosphenidans or other extinct crown-mammals.
* Though note that more recent phylogenetic studies have mostly destroyed the relationships used to support this possibility. See comments for more.
Caption: Lee & Shine (1998) discussed several cases in squamate phylogeny where oviparity might have re-evolved within a viviparous clade (the example here concerns anguid lizards). In at least some of these cases, however, the topologies that support such transitions are not especially robust and have been overturned by more recent studies (1998 is a long time ago when it comes to squamate phylogeny).
The problem of tiny multituberculates. Let’s say that extinct non-therians, like multituberculates, did lay eggs in monotreme fashion. A possible problem with this idea (is it a problem? Hold that thought…) is that multituberculate eggs – and perhaps those of other non-therian mammals too – would be almost unbelievably tiny. Some multituberculates were so small as adults, and have such atypically narrow pelvic girdles, that an egg – assuming it had a circular cross-section – would have to be between 2 and 4 mm wide (not a typo) in the smaller members of the group (Kielan-Jaworowska 1979, Kielan-Jaworowska et al. 2004). Such eggs would be “smaller than any known cleidoic egg” if they were round (Kielan-Jaworowska et al. 2004, p. 299), but – if egg-shaped or elongate – about similar in size to grains of rice (‘cleidoic’ means enclosed within a shell). Similar things might hold true for various other extinct synapsid groups – some of which were similarly tiny – though this is harder to evaluate since their pelvic girdles tend not to be known. They probably weren’t as narrow in the pelvis as multituberculates are, however. This inferred tiny egg size led Kielan-Jaworowska (1979) to favour viviparity for multituberculates.
Caption: at left, the pelvic girdle of the small multituberculate Kryptobaatar dashzevegi in (at left) dorsal view and (at right) posterior view, as figured by Kielan-Jaworowska (1979). The published version of Kielan-Jaworowska (1979) has these diagrams mislabelled. At right, a Kryptobaatar skeleton photographed in the Musee d'Histoire Naturelle, Brussels. This was a large-eyed, superficially rodent-like animal. Images: Kielan-Jaworowska (1979); Ghedogedo, CC BY SA 3.0 (original here).
At this point you might be wondering if a cleidoic egg this small is physiologically and mechanically possible (the surface area : volume ratio would obviously result in a proportionally huge surface). I’m not about to do the maths to find out, but – if we assume an ovoid or semi-cylindrical form for such an egg – then a width of 2 or 3 or 4 mm wide results in a length of something like 6 mm long at least… which is about the same as the smallest eggs produced by modern hummingbirds (bee hummingbird eggs are about 7 mm long). Tiny gecko eggs are about 6.5 mm long, but they’re round.
It has also been noted that the narrow multituberculate pelvis (and concomitant tiny inferred egg size) is quite different from the condition in monotremes, where the pelvic opening is relatively wide and U-shaped. The idea here is that -- if we were to predict the production of monotreme-like eggs in mammals like multituberculates – we would surely expect a wider pelvic canal more like that of monotremes. But would we?
Let’s shrink some monotremes. Well, here’s the thing. Despite having a wider pelvic canal than a small multituberculate, even monotremes don’t lay eggs that big. Those of the platypus are about 11 mm wide, those of short-beaked echidnas are about 14 mm wide. The multituberculates suggested by Kielan-Jaworowska (1979) to be incapable of producing only the tiniest eggs were small animals with head + body lengths of 10 cm or less; they were much smaller than living monotremes, where head + body length is (for females) between 30 and 40 cm.
Caption: I’m surprised to find that I don’t own all that many echidna-themed images, but here are two. The painting is at Marwell Wildlife; the skeleton of Tachyglossus is (or was?) on show at the Hunterian Museum and Art, Gallery, Glasgow. Images: Darren Naish.
If we then imagine a monotreme that’s shrunk down to the size of a small multituberculate – and assume an isometric reduction in egg size (read on) – we end up with imaginary monotreme eggs that are around 3.5 mm wide… about the same size as the projected tiny multituberculate eggs. In other words, it might be that any oviparous mammal similar in size to those small multituberculates would have laid similarly small eggs, and the probable smallness of the eggs isn’t a factor which demonstrates that they couldn’t exist. Caveat: my assumption there of a simple, isometric scaling of egg size is dodgy, since the eggs of some small tetrapods haven’t scaled this way during evolution (the eggs of small birds, for example, are often relatively large). It should be noted that Hopson (1973) proposed that miniaturisation of eggs was an important step in the evolution of mammalian viviparity, since miniaturised eggs meant shorter incubation time and less nourishing of the embryo within the egg, and hence more pressure for parental provisioning of ‘fetus-like’ hatchlings.
In any case, things aren’t necessarily less surprising if we imagine that small multituberculates were viviparous. Again, the dimensions and shape of the multituberculate pelvis mean that live babies would have to be tiny. Kielan-Jaworowska (1979) worked out that the head of a newborn Kryptobaatar couldn’t be wider than 3.4 mm, the resulting ratio of newborn skull width to adult skull width being similar to that of some small living marsupials (like the dasyurid Antechinus). The babies would therefore be nidicolous little things maybe 15 mm long or so. Which is small, but not absurdly small when you consider than the newborns of some Antechinus species are 4 or 5 mm long (again, not a typo) (Nowak 1999).
Barring the almost unbelievable possibility that these animals reproduced in some other way (like budding, or chest-bursting… no, I’m not being serious, I think), we seem to be stuck with the possibility that small multituberculates like Kryptobaatar were either laying tiny eggs – among the smallest cleidoic eggs ever in history, but plausibly similar in size to other tiny cleidoic eggs – or were giving birth to tiny live babies, which were small but not absurdly so. In keeping with the more flexible regime suggested by Guillette & Hotton (1986), it’s also possible that the small multituberculates seen as a problem for the oviparity idea by Kielan-Jaworowska (1979) were unusual in reproductive style compared to other members of the group: maybe they evolved viviparity while other multituberculates were oviparous. Multituberculates, incidentally, were around for over 100 million years, so there’s the scope within this one group for all sorts of evolutionary innovations and deviations. Likewise for several other groups.
Those Kayentatherium babies. There’s one final thing to say on this issue, and this concerns the juvenile stem-mammals that have been discovered so far. In 2018, Hoffman & Rowe (2018) reported an amazing fossil assemblage from the Lower Jurassic of Arizona: an adult specimen of the tritylodontid Kayentatherium wellesi preserved with the remains of 38 tiny juveniles, all of which are ‘reptile-like’ in being proportioned much like adults.
Caption: zombie Kayentatherium considers her tiny babies. I haven’t yet done any tritylodontid imagery myself…. Image: (c) University of Texas (original here).
Unfortunately, there’s no eggshell preserved, and no direct evidence on whether those babies were born live or hatched from eggs. Both possibilities exist, and the fact that the babies are ‘reptile-like’ in terms of proportions and size relative to the parent doesn’t help either way. I note, however, that the authors use the term ‘clutch’ when referring to this assemblage, and thus imply that the babies did hatch from eggs. Tritylodontids are not mammals, for those of you who don’t know, but are close to their origin (here I’m using ‘mammal’ to be synonymous with the clade termed Mammaliaformes by most experts… ugh). If the ‘reptilian’ style of producing numerous small babies was present this close to mammal ancestry, perhaps it was present throughout at least some non-therian lineages. Whether this was so or not, it doesn’t – I say again – help us in determining which of the relevant lineages were oviparous or viviparous.
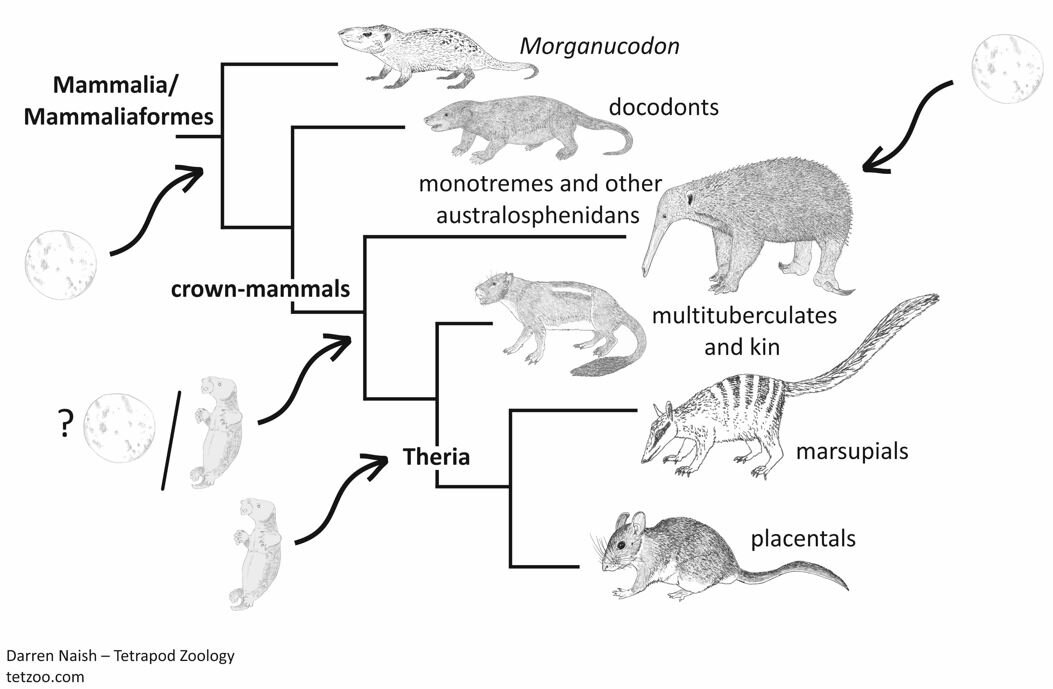
Caption: a highly speculative depiction of how oviparity and viviparity might have been distributed across mammals. Image: Darren Naish.
Here’s where I want to see interesting insight and discussion. As expressed in this article, this is a surprisingly under-discussed area, yet it has major implications for how we imagine non-therian mammals and other extinct synapsids. What we do think? Was oviparity rare, or widespread, or was there really the variation suggested by Guillette & Hotton (1986)? I await your thoughts.
Mesozoic mammals, monotremes and other synapsids haven’t been covered at TetZoo all that much; they’re simply among those groups I’ve never got around to writing much about. It’s one of those things I aim to rectify in time. For relevant previous articles, see…
Survivors, diggers, herbivores, first giant terrestrial vertebrates: the caseids, July 2007
The answers we seek: on ‘goodbyes’, the necks of caseids, and weird mystery sauropods, July 2007
Perentie tries to swallow echidna. Echidna too spiky, Perentie gets horribly injured. Dies., December 2009
Late Cretaceous Animals of Romania's Haţeg Island--a More Complex View, September 2015
The Stem-Mammals--a Brief Primer, September 2016
Refs - -
Guillette, L. & Hotton, N. 1986. The evolution of mammalian reproductive characteristics in therapsid reptiles. In Hotton, N., MacLean, P. D., Roth, J. J. & Roth, E. C. (eds) The Ecology and Biology of Mammal-Like Reptiles. Smithsonian Institution Press, Washington and London, pp. 239-250.
Hoffman, E. A. & Rowe, T. B. 2018. Jurassic stem-mammal perinates and the origin of mammalian reproduction and growth. Nature 561, 104-108.
Hopson, J. A. 1973. Endothermy, small size and the origin of mammalian reproduction. American Naturalist 107, 446-452.
Kielan-Jaworowska, Z. 1979. Pelvic structure and nature of reproduction in Multituberculata. Nature 277, 402-403.
Kemp, T. 1982. Mammal-like Reptiles and the Origin of Mammals. Academic Press, London.
Lee, M. S. Y. & Shine, R. 1998. Reptilian viviparity and Dollo’s law. Evolution 52, 1441-1450.
McLoughlin, J. C. 1979. Archosauria: A New Look at the Old Dinosaur. Penguin Books, London.
Sharman, G. B. 1970. The biology of sex in marsupials. Australian Journal of Science 32, 307-314.
Surget-Groba, Y., Heulin, B., Guillaume, C. P., Thorpe, R. & Kupriyanova, L. 2001. Intraspecific phylogeography of Lacerta vivipara and the evolution of viviparity. Molecular Phylogenetics and Evolution 18, 449-459.